Methods for producing hydrogen in industrial conditions
Extraction by methane conversion
... Water in a vapor state, preheated to 1000 degrees Celsius, is mixed with methane under pressure and in the presence of a catalyst. This method is interesting and proven; it should also be noted that it is constantly being improved: new catalysts, cheaper and more effective, are being searched for.
Consider the most ancient method of producing hydrogen - coal gasification
... In the absence of air access and a temperature of 1300 degrees Celsius, coal and water vapor are heated. Thus, hydrogen is displaced from water, and carbon dioxide is obtained (hydrogen will be at the top, carbon dioxide, also obtained as a result of the reaction, is at the bottom). This will be the separation of the gas mixture, everything is very simple.
Obtaining hydrogen by electrolysis of water
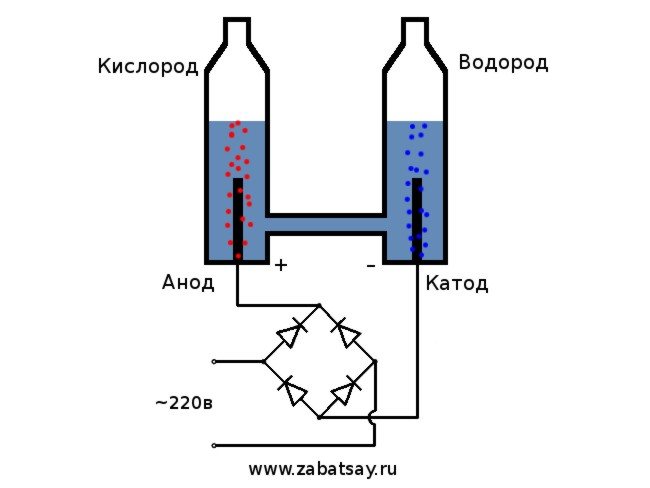
is considered the simplest option. For its implementation, it is necessary to pour a soda solution into the container, and also place two electrical elements there. One will be charged positively (anode) and the other negatively (cathode). When current is applied, hydrogen will go to the cathode and oxygen to the anode.
Obtaining hydrogen by the method partial oxidation
... For this, an alloy of aluminum and gallium is used. It is placed in water, which leads to the formation of hydrogen and alumina during the reaction. Gallium is necessary for the reaction to occur in full (this element will prevent aluminum from oxidizing prematurely).
Recently acquired relevance method of using biotechnology
: under the condition of a lack of oxygen and sulfur, chlamydomonas begin to intensively release hydrogen. A very interesting effect that is now being actively studied.

Do not forget another old, proven method of hydrogen production, which consists in using different alkaline elements
and water. In principle, this technique is feasible in a laboratory setting with the necessary safety measures in place. Thus, in the course of the reaction (it proceeds with heating and with catalysts), a metal oxide and hydrogen are formed. It remains only to collect it.
Get hydrogen by interaction of water and carbon monoxide
possible only in an industrial environment. Carbon dioxide and hydrogen are formed, the principle of their separation is described above.

How to get hydrogen safely at home?
Such questions are touching, because it seems to a common man in the street that it is quite simple to get hydrogen, and yet this, although it can be done under normal conditions, is still quite dangerous. The first thing you need to know is that you need to do such experiments only in the open (outdoors) air, since hydrogen is a very, very light gas (about 15 times lighter than standard air) and it will accumulate near the ceiling, forming a highly explosive mixture. If all the necessary measures are taken to prevent problematic moments, then it is possible to carry out the reaction of the interaction of alkali and aluminum.
We take a flask (best of all) or a 1/2 liter glass bottle, a cork (in the middle of the hole), a tube for removing hydrogen, 10 grams of aluminum and vitriol (copper), table salt (about 20 grams), water in an amount 200 ml. and a ball (rubber) for collecting hydrogen. We buy vitriol in gardening shops, and beer cans or wire may well act as aluminum raw materials. Of course, the enamel is preliminarily removed by firing, you need pure aluminum, without impurities.
For 10 grams of vitriol, 100 ml of water is taken, respectively, and a second solution is prepared - for 20 grams of salt, 100 ml of water will go. The shade of the solutions will be as follows: vitriol - blue, salt - colorless. Then we mix everything together and we get such a greenish solution. Pre-prepared aluminum is added to it. The mixture will begin to foam - this is hydrogen. Aluminum replaces copper, and you can see it with your own eyes by the bloom of a reddish tint on aluminum raw materials. A whitish suspension appears, it is here that you can begin to collect the hydrogen we need.
In the process, additional heat is obtained; in chemistry, such a process is referred to as exothermic. It is clear that if the process is not controlled, then something like a geyser will turn out, which will spit out portions of boiling water, so the initial concentration must be controlled. For this, a plug with a tube is used to safely remove the hydrogen to the outside. The diameter of the tube, by the way, should not exceed 8 millimeters in any way. The collected hydrogen can inflate the balloon, which will be much lighter than the surrounding air, which means it will allow it to rise up. Honestly, such experiments must be practiced extremely carefully and carefully, otherwise injuries and burns cannot be avoided.
THE INVENTION HAS THE FOLLOWING ADVANTAGES
The heat obtained from the oxidation of gases can be used directly on site, and hydrogen and oxygen are obtained from the disposal of waste steam and process water.
Low water consumption when generating electricity and heat.
The simplicity of the way.
Significant energy savings as it is spent only on warming up the starter to the established thermal regime.
High productivity of the process, because dissociation of water molecules lasts tenths of a second.
Explosion and fire safety of the method, because in its implementation, there is no need for containers for collecting hydrogen and oxygen.
During the operation of the installation, water is repeatedly purified, being converted into distilled water. This eliminates sediments and limescale, which increases the service life of the installation.
The installation is made of ordinary steel; with the exception of boilers made of heat-resistant steels with lining and shielding of their walls. That is, no special expensive materials are required.
The invention can find application in
industry by replacing hydrocarbon and nuclear fuel in power plants with cheap, widespread and environmentally friendly water, while maintaining the power of these plants.
Alternative view
The utility model relates to electrochemistry and, more specifically, to hydrogen energy and can be useful for obtaining a fuel mixture with a high hydrogen content from any aqueous solutions.
Known devices for direct electrochemical decomposition (dissociation) of water and aqueous solutions into hydrogen and oxygen by passing an electric current through the water. Their main advantage is their ease of implementation. The main disadvantages of the known hydrogen generator-prototype device are low productivity, significant energy consumption and low efficiency. The theoretical calculation of the required electricity for the production of 1 m3 of hydrogen from water is 2.94 kWh, which still makes it difficult to use this method of hydrogen production as an environmentally friendly fuel in transport.
—
The closest device (prototype) by design and the same purpose to the claimed utility model by a combination of features is a well-known electrolyzer - the simplest hydrogen generator containing a hollow chamber with an aqueous solution (water), electrodes placed in it, and a source of electricity connected to them (book. Chemical encyclopedia ", v. 1, m., 1988, p. 401)
The essence of the prototype - the well-known hydrogen generator consists in the electrolytic dissociation of water and aqueous solutions under the action of an electric current on H2 and O2.
Lack of prototype consists in low hydrogen productivity and significant energy consumption.
The purpose of the present invention is the modernization of the device to improve its energy efficiency
Technical result, of this utility model consists in the technical and energetic improvement of the known device, which is necessary to achieve this goal.
Specified technical result is achieved by the fact that the known device containing a hollow chamber with an aqueous solution, electrodes placed in water, an electricity source connected to them, is supplemented with capillaries placed vertically in water, with upper ends above the water level, and the electrodes are made flat, one of which is placed under the capillaries, and the second electrode is made of mesh and is located above them, and the power source is made of high-voltage and adjustable in amplitude and frequency, and the gap between the ends of the capillaries and the second electrode and the parameters of the electricity supplied to the electrodes are selected according to the condition of ensuring maximum hydrogen productivity, and the regulators capacity is the voltage regulator of the said source and the regulator of the gap between the capillaries and the second electrode, and the device is also supplemented by two ultrasonic generators, one of which is located under the lower end of these capillaries and the second - above their upper end, and the device The unit is also supplemented with an electronic dissociator of activated water mist molecules containing a pair of electrodes located above the liquid surface, with their planes perpendicular to the liquid surface, and electrically connected to an additional electronic generator of high-voltage high-frequency pulses with an adjustable frequency and duty cycle, in the frequency range overlapping the resonant excitation frequencies evaporated molecules of a liquid and its ions.
Promotional video:
DESCRIPTION OF THE DEVICE IN THE STATIC
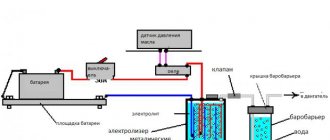
Device for producing hydrogen from water (fig. 1) consists of a dielectric container 1, with an aqueous solution of liquid 2 poured into it, of a finely porous capillary material 3, partially immersed in this liquid and pre-moistened in it. This device also includes high-voltage metal electrodes 4, 5, placed at the ends of the capillaries 3, and electrically connected to the terminals of a high-voltage regulated source of a constant-sign electric field 10, and one of the electrodes 5 is made in the form of a perforated-needle plate, and is positioned movably above the end of the capillaries 3, for example, parallel to it at a distance sufficient to prevent electrical breakdown to the wetted wick 3. Another high-voltage electrode 4 is placed in the liquid parallel to the lower end of the capillary, for example, porous material 3 The device is supplemented with two ultrasonic generators 6, one of which is located in the liquid 2, almost at the bottom of the container 1, and the second is located above the liquid level, for example mesh electrode 5.
The device also contains an electronic dissociator of molecules of activated water mist, consisting of two electrodes 7,8, located above the surface of the liquid, with their planes perpendicular to the surface of the liquid, and electrically connected to an additional electronic generator 9 high-voltage high-frequency pulses with adjustable frequency and duty cycle, in the range frequencies that overlap the resonance frequencies of excitation of the evaporated molecules of the liquid and its ions.The device is also supplemented with a bell 12, located above the tank 1 - a collection gas collector 12, in the center of which there is an outlet pipe for withdrawing fuel gas and H2 to consumers. In essence, the device assembly containing the electrodes 4,5 from the high voltage units 10 and the capillary assembly 3 4, 5, 6 is a combined device of an electroosmotic pump and an electrostatic evaporator of liquid 2 from container 1 ... Unit 10 allows you to regulate the duty cycle of pulses and the intensity of a constant electric field from 0 to 30 kV / cm. The electrode 5 is made of a metal perforated or mesh to provide the possibility of unhindered passage of the formed water mist and fuel gas from the end of the capillaries 3. The device has regulators and devices for changing the frequency of pulses and their amplitude and duty cycle, as well as for changing the distance and position of the electrode 5 relative to the surface of the capillary evaporator 3 (they are not shown in Fig. 1).
DESCRIPTION OF THE DEVICE OPERATING DEVICE (FIG. 1)
First, an aqueous solution is poured into the container 1, for example, activated water or a water-fuel mixture (emulsion) 2, the capillary 3-porous evaporator is pre-moistened with it. Then, a high-voltage voltage source 10 is turned on and a high-voltage potential difference is supplied to the capillary evaporator 3 through the electrodes 4.5, and the perforated electrode 5 is placed above the surface of the end face of the capillaries 3 at a distance sufficient to prevent electrical breakdown between the electrodes 4.5. As a result, along the fibers of capillaries 3 under the action of electroosmotic and, in fact, electrostatic forces of a longitudinal electric field, water clusters are partially ruptured and sorted in size, absorbed into capillaries 3. Moreover, dipole polarized liquid molecules unfold along the electric field vector and move from the container towards the upper end capillaries 3 to the opposite electric potential of electrode 5 (electroosmosis). Then they, under the action of electrostatic forces, are torn off by these electric field forces from the surface of the end of capillary 3 - essentially an electroosmotic evaporator and turn into a partially dissociated polarized electrified water mist. This water mist above the electrode 5 is then also intensively treated with a pulsed transverse high-frequency electric field created between the transverse electrodes 7,8 by an electronic high-frequency generator 9. In the process of intense collision of evaporated dipole molecules and water clusters above the liquid with air and ozone molecules, electrons in the ionization zone between the electrodes 7, 8, an additional intensive dissociation (radiolysis) of the activated water mist occurs with the formation of a fuel combustible gas. Further, this obtained fuel gas flows independently upward into the gas-collecting bell 12 and then through the outlet 13 is supplied to consumers for preparing a synthetic fuel mixture, for example, into the intake tract of internal combustion engines and supplying it to the combustion chambers of a motor vehicle. The composition of this combustible gas includes molecules of hydrogen (H2), oxygen (O2), water vapor, fog (H2O), as well as activated organic molecules evaporated as part of other hydrocarbon additives. Previously, the operability of this device was shown experimentally and it was found that the intensity of the process of evaporation and dissociation of molecules of aqueous solutions significantly depends and changes depending on the parameters of the electric field of the sources9,10. (Intensity, power), on the distance between the electrodes 4, 5, on the area of the capillary evaporator 3, depending on the type of liquid, the size of the capillaries and the quality of the capillary material 3.The regulators available in the device allow you to optimize the performance of the fuel gas depending on the type and parameters of the aqueous solution and the specific design of this electrolyzer. Since in this device an aqueous solution of a liquid intensively evaporates and partially dissociates into H2 and O2, under the action of capillary electroosmosis, and ultrasound, and then additionally actively dissociates due to intense collisions of molecules of the evaporated aqueous solution by means of an additional transverse resonant electric field, such a device for producing hydrogen and fuel gas consumes little electricity and therefore is much more economical by tens of hundreds of times more economical than known electrolysis hydrogen generators.
CLAIM
An ultrasonic device for producing hydrogen from any aqueous solution, containing a container with an aqueous solution, metal electrodes placed in it, and a source of electricity connected to them, characterized in thatit is supplemented by capillaries placed vertically in this chamber, with their upper ends above the level of the aqueous solution, and one of the two electrodes is placed in the liquid under the capillaries, and the second electrode is made movable and gridded and placed above them, and the power source is made of high-voltage and adjustable in amplitude and frequency, and the device is also supplemented by two ultrasonic generators, one of which is located under the lower end of these capillaries and the second is located above their upper end, and the device is also supplemented with a resonant electronic dissociator of activated water mist molecules containing a pair of electrodes located above the liquid surface, with their planes, perpendicular to the surface of the liquid, and electrically connected to an additional electronic generator of high-voltage high-frequency pulses with an adjustable frequency and duty cycle, in the frequency range containing the resonant excitation frequencies of the evaporated liquid molecules and its ions.
CLAIM
Method for producing hydrogen and oxygen from water vapor
, including passing this steam through an electric field, characterized in that they use superheated water steam with a temperature
500 - 550 o C
, passed through a high voltage direct current electric field to dissociate vapor and separate it into hydrogen and oxygen atoms.
I have long wanted to do a similar thing. But further experiments with a battery and a pair of electrodes did not reach. I wanted to make a full-fledged apparatus for the production of hydrogen, in quantities to inflate a balloon. Before making a full-fledged apparatus for electrolysis of water at home, I decided to check everything on the model.
The general scheme of the electrolyzer looks like this.

This model is not suitable for full daily use. But we managed to test the idea.
So I decided to use graphite for the electrodes. An excellent source of graphite for electrodes is the trolley bus collector. There are plenty of them lying around at the end stops. It must be remembered that one of the electrodes will collapse.


We saw and finalize with a file. The intensity of electrolysis depends on the strength of the current and the area of the electrodes.


Wires are attached to the electrodes. The wires must be carefully insulated.


For the case of the electrolyzer model, plastic bottles are quite suitable. Holes are made in the cover for pipes and wires.


Everything is thoroughly coated with sealant.


Cut-off bottle necks are suitable for connecting two containers.


They need to be joined together and the seam must be melted.


The nuts are made from bottle caps.


Holes are made in two bottles at the bottom. Everything is connected and carefully filled with sealant.


We will use a 220V household network as a voltage source.I want to warn you that this is a rather dangerous toy. So, if you do not have sufficient skills or there are doubts, then it is better not to repeat. In the household network, we have an alternating current, for electrolysis it must be straightened. A diode bridge is perfect for this. The one in the photo was not powerful enough and quickly burned out. The best option was the Chinese MB156 diode bridge in an aluminum case.


The diode bridge gets very hot. Active cooling will be required. A cooler for a computer processor is perfect. A junction box of a suitable size can be used for the enclosure. Sold in electrical goods.


Several layers of cardboard must be placed under the diode bridge.


The necessary holes are made in the cover of the junction box.


This is what the assembled unit looks like. The electrolyzer is powered from the mains, the fan is powered by a universal power source. A baking soda solution is used as an electrolyte. Here it must be remembered that the higher the concentration of the solution, the higher the reaction rate. But at the same time, heating is also higher. Moreover, the reaction of sodium decomposition at the cathode will contribute to the heating. This reaction is exothermic. As a result, hydrogen and sodium hydroxide will be formed.


The device in the photo above was very hot. It had to be turned off periodically and wait until it cools down. The heating problem was partially solved by cooling the electrolyte. For this I used a tabletop fountain pump. A long tube runs from one bottle to another through a pump and a bucket of cold water.


The relevance of this issue today is quite high due to the fact that the sphere of using hydrogen is extremely extensive, and in its pure form it is practically not found anywhere in nature. That is why several techniques have been developed that allow the extraction of this gas from other compounds through chemical and physical reactions. This is discussed in the article above.
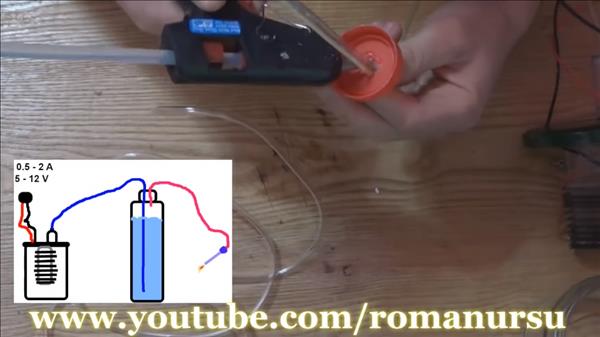
The guy made an installation for producing hydrogen
Roman Ursu. In this video I wanted to show how you can make a small generator from 10 shaving blades that will extract hydrogen from water. To get started, you need a power supply unit from 5 to 12 volts, current strengths from 0.5 to 2 amperes. Copper wires, glass jar with a sealed screw cap. A plastic bottle, a piece of a plastic ruler. Two droppers. 10 blades. Edible salt. Tools: soldering iron, glue gun, stationery knife.

Products for inventors