What happens if you pour anti-freeze into the cooling system. Is it okay to risk a car like that?
The question demanded by my readers, I must say that novice drivers are interested in it. To be honest, guys, I do not urge anyone to take such a risk, but it is clear that antifreeze or antifreeze are much more expensive than anti-freeze, and the compositions seem to be almost the same! Both liquids do not freeze at low temperatures! So why overpay? After all, before they just poured water into the engine cooling system - so why not pour an anti-freeze? What will happen? To all curious my today's article ...

THE CONTENT OF THE ARTICLE
If you dig a little into history, then really some 30 years ago, very often in the cooling system filled with plain water, it was quite normal practice. But it is worth noting that other fluids were other, less perfect chtoli, if not "tougher" - they were generally far from those fluids that we are now pouring in, for example - oils (motor and transmission, antifreeze and antifreeze, respectively) - so a major overhaul once a year was then a normal picture.
A lot of "water has flowed under the bridge" since that time, now there are other technologies, other engines and fluids. The lubrication is so perfect that it allows the motor to walk 500,000 kilometers (and maybe more) with a timely replacement, without "Capital"... But such motors should be really cool, high speed - fast heating - there should be rapid cooling. If our power unit runs for a long time, then the cooling system should not, fail, that is, fluids for it must be the right coloras well as the required tolerances! Hypothetically, it is possible to pour water into the cooling system, but this is not worth doing, for several reasons.
What is non-freezing
Before proceeding directly to the issue under consideration, you need to understand the differences between fluids - anti-freeze and antifreeze (coolant, antifreeze).
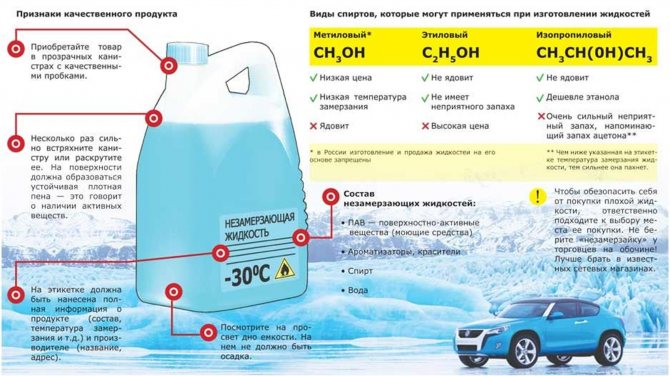
Non-freezing, as the name suggests, is capable of not freezing at low temperatures, and its task is to clean the windshield of a car at negative ambient temperatures. Antifreeze consists of the simplest elements - alcohol, water and various additives (aromatic, dyes, and so on). Since water contains alcohol, it does not freeze even at low temperatures, and the higher the concentration of alcohol in the anti-freeze, the lower temperatures it is able to retain its properties.
Anti-freeze can be divided into three types, depending on the alcohol that is used in them:
- Isopropyl. Harmful alcohol for the human body, which in no case should be taken orally. Even if you drink 100 ml of such alcohol, it can be fatal;
- Methanol. Alcohol, also poisonous to the human body, has a simple monoatomic composition;
- Bioethanol. Relatively harmless to the human body alcohol, close in its characteristics to the usual medical alcohol. Used in good anti-freeze applications.
Please note: At the end of the 90s, ordinary medical alcohol was actively used in antifreeze. But due to the massive consumption of this liquid "inside", which was cheaper than alcoholic beverages due to the lack of excise stamps, over time, such non-freezing was banned.
One of the three types of alcohols mentioned above is mixed with water, the liquid is colored with an ordinary dye, some compounds are added to improve the quality of cleaning, and an anti-freeze is obtained from this.
Can water be added to the cooling system?
Sure you may! Why not - that's just your long-running engine, after a few thousand kilometers, will require repair! And this is natural. There are several reasons for this:
- Water oxidizes metal walls - radiators, pipes, engine block, etc., which will eventually lead to rusting.We will provoke unnecessary heating of the unit.
- After rust can precipitate, which will lead to clogging of the cooling channels. The engine will overheat.
- Water boils already at 100 degrees Celsius, which cannot effectively cool the motor.
- Water freezes in winter - if "missed", it will simply rupture the system.
- Some Japanese and European cars simply will not start with water, they have a sensor that detects the quality of the liquid.
Now, from personal experience, I myself know how the radiator gets rusty and clogged after using water, I passed it on my Moskvich 2140, very many years ago. So there is no need to pour! It is not right.
Filling the heating system with liquid
Experts say there are two ways to fill a closed system with liquid:
- using a pump. The fluid should be pumped from a drum or tank. In this case, it is imperative to use a pressure gauge. The injection should be stopped as soon as the pressure reaches 1.5 bar (we are talking about a private house no higher than 2 floors). The pump is connected to the make-up or drain pipe through a check valve. In this case, all air valves should be opened;
- manually. You will have to pour the finished coolant through the air vent on the riser (this is the highest point of the system). Reviews claim, that this method is more laborious. As in the first case, it is necessary to connect a pressure gauge and open all air valves. Manual air injection and bleeding must be done until you see the desired pressure on the pressure gauge.
From this video you will learn how to properly fill the heating system with antifreeze in a private house:
Non-freezing - composition
Ever wondered what it is made of? What is its composition? In fact, it's just:
- Alcohol - methyl (or isopropyl) is used, poisonous, you can not drink them! Previously, of course, there were compositions based on ethyl alcohol, but our compatriots drank them for a nice thing, so now only - "methyl".


- Water - here its greater volume, usually about 60 - 70%, it all depends on the concentration and temperature threshold.
- Surfactants - detergents, fight with poplar buds, flies, on the windshield, etc.
- Dyes - it is they who paint the "non-freeze" in blue, so similar to antifreeze.
- Flavors - make the anti-freeze a pleasant smell - herringbone, banana, krubnik, etc.
So the whole composition, as you can see, is rather "primitive", but in fact it is no longer needed - for washing the glass.
Non-freezing
This is a liquid that is designed not to freeze at different temperatures, so that even in minus indicators, it washes your windshield.
The composition of the anti-freeze is simple - alcohol + water + aromatic or color additives. Alcohol prevents this liquid from freezing, and the more it is, the lower the freezing point.


Only three types of alcohols are used in the washer fluid:
- Isopropyl is a technical alcohol, poisonous, if you drink about 100-200 ml, it leads to lethal consequences.
- Methanol (methyl) alcohol, a simple monohydric composition, is also poisonous, if you use about 100 ml it is fatal.
- Bioethanol - alcohol, harmless to the body, similar to medical alcohol, has been used in the EUROPEAN UNION since 2012 as a substitute for toxic competitors.
For the sake of fairness, it is worth noting that they used to use medical or food alcohol, but since our "people's" motorists began to get alcohol from it and then drink it, they were banned.
To these alcohols, ordinary tap water is added, as well as blue flavors and dyes, sometimes compounds are added that help wash away glass contaminants, for example, from tree buds, midges, etc. This is how the non-freezing liquid turns out, which we pour into the washer reservoir with you. Nothing beyond natural.
Antifreeze - antifreeze - compounds
To be honest, many manufacturers keep the formula a closely guarded secret. Of course, everyone knows the approximate composition, but the exact one is a secret. If we take the average, then:
- Ethylene glycol (or higher alcohol) is the main component. Not poisonous, but you can't drink it!


- Additives that protect rubber products - from swelling, destruction.
- Additives that protect the engine from overheating, antifreeze (antifreeze) has a higher boiling point than water.
- Compounds that protect metal parts from oxidation, if you like, these are a kind of oily liquids.
- Of course, water, where is it without it.
- Dyes that are specially added to differentiate concentration and properties.
Types of antifreeze
The market for this particular product is very extensive. Recently, due to the increased demand for anti-freeze products, manufacturers have greatly expanded their assortment.
Non-freezing liquids are made on the basis of various chemical compounds:
- Glycerin;
- Ethylene glycol;
- Propylene glycol;
- Bischofite brine;
- Saline solution.
The most common household "non-freezing" products are made on the basis of aqueous solutions of ethylene glycol, glycerin and propylene glycol. Since these substances are highly aggressive, special components are added to them - additives.
The purpose of which is to prevent damage, corrosion, scale and foaming.
- Ethylene glycol is the most popular among our consumers. Their main advantage is their low price. But at the same time it is the most toxic non-freezing liquid, the use of which in double-circuit boilers is prohibited, due to the high probability of entering the water supply system, which is dangerous to human health. It should be borne in mind that when the boiling point rises above 110 degrees, ethylene glycol gives a precipitate that can damage some elements of the system.
- Propylene glycols are similar in properties to the first type, but at the same time they are harmless and safe. Most of the manufacturers recommend them.
- Glycerin is absolutely non-toxic and environmentally friendly, providing maximum protection against corrosion. It does not increase in volume when it goes into a solid state, and it is enough to simply heat it up to start the system.
- Antifreezes based on a natural bischofite solution have unique physical and chemical properties. Low freezing point and high boiling point, as well as greater heat capacity and heat transfer than water, which is not typical for most of these products.
- Salt coolants are produced on the basis of solutions of mineral salts (magnesium, calcium, sodium and their compounds). A significant disadvantage of these fluids is their high corrosiveness to equipment.
Antifreezes are sold either already diluted and ready for use (experts recommend using a coolant with a freezing temperature of -20 to -25 degrees), or in the form of concentrates, and then the solution must be prepared independently.
An example of diluting ethylene glycol fluids. They are of two types:
- With a freezing threshold not higher than -30 degrees (then, to come to a freezing point of -25, the mixture must be diluted with distilled water in a ratio of 9: 1);
- With a freezing threshold not higher than -65 degrees (to get a freezing threshold of -25, antifreeze is mixed with water in proportions of 6: 4).


So what will happen if you pour an anti-freeze?
So we come to the most interesting thing - the denouement, so what will happen if you pour an anti-freeze liquid into the cooling system?


Let's imagine this hypothetically, because I'm not urging you to do this:
- When the engine warms up, methyl alcohol will quickly begin to evaporate, and as we remember, it is "poisonous", that is, you will breathe it in the car, because the stove sucks in air from the front. Poisoning is possible.
- This "slurry", which was left without alcohol, can simply freeze even at sub-zero temperatures.
- The surfactants that are in the composition will begin to foam wildly (the pump will begin to whip it), in fact, these are detergents - from the radiator to the cooling system and the tank, foam will climb, which will quickly freeze at minus, forming plugs and "airing"! Overheating of the engine is provided.


- Perhaps he will quickly eat rubber gaskets and other sealing elements - who knows what is in the composition.
- The walls of the engine and pipes will begin to oxidize from the same surfactants.
- If engine oil gets into the engine system, the anti-freeze will not be able to "overcome" it, it is not designed to remove oil. But antifreeze or antifreeze would simply absorb the oil into its composition.
If you do not want to QUICKLY "ditch" your engine, do not pour anti-freeze into the cooling system, it is simply dangerous! It is not designed for such working conditions.
On the way, there may be a need to fill the cooling system with another liquid other than antifreeze, for example - the radiator has been punctured, and the system simply leaked - then just fill in water, this is much more correct. But then, when we arrived at our destination, fix the cooling system, flush it, and fill only with antifreeze - antifreeze, this is the most correct way.
Don't do nonsense - NEVER put anti-freeze into the cooling system. THIS IS FULL BAD! The failure of your unit is almost guaranteed, not immediately, but after some time.
I hope I explained in a popular way, if it's not entirely clear, watch the video - just about the difficult.
This concludes, read our car site.
(8 votes, average: 4,63 out of 5)
Rules for the use of antifreeze
Filling the system with new mixture can only be done after it has been cleaned of the previous "filler" and checked for leaks and cracks. Remember that you need to achieve complete tightness to avoid operational problems.
If necessary, carry out maintenance and replace worn parts.
When you understand that the batteries and pipes are in order, you can proceed to the most time-consuming procedure - pouring antifreeze. It is important to do this immediately after preparing the mixture (antifreeze, as you already know, will need to be diluted with water) so that it remains homogeneous.
Remember that non-freezing is a rather capricious chemical "cocktail" that requires a special approach.
- a trial run of the system must be done with minimum power. Further, the turnover will need to be increased up to the norm;
- antifreeze can only be poured into single-circuit boilers;
- literally made to fill them with antifreeze. If you go to optimize the operation of an electrical installation by adding an anti-freeze liquid to it, this can lead to serious overheating;
- strictly follow the instructions on the antifreeze container and the recommendations from the manufacturer of your heating equipment. Otherwise, you may get into trouble due to clogged filters. This leads to a decrease in heat transfer due to breakdown of pumping systems.
Is it possible to pour an anti-freeze solution instead of antifreeze? A question the answer
Often novice drivers ask if it is possible to fill an anti-freeze instead of antifreeze. Everyone has their own reasons for this. Most often, non-standard fluids are used when it is necessary to get to the nearest settlement, but sometimes, people simply add the first available liquid to the antifreeze, without thinking about the consequences. Then they have to capitalize on the power unit. A rarer case is the use of an anti-freeze liquid for glass in order to save. This approach is not only wrong, but also dangerous for the motor. Well, the amount saved can be considered very conditional.
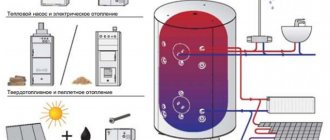
Recommendations for the selection and operation of heat carriers - which one is better to choose
None of the manufacturers of heat carriers will refute the fact that in the case of stable operation of the heating system in winter, it is water that is the best option, which heat carrier to choose for heating. It is better if it is a special distilled liquid with modifying additives, as mentioned earlier. Homeowners who consider buying store water a waste of money usually do their own preparation, softening it, and fitting the system with the right filters.


If it was decided to use non-freezing coolants, it is important to have information on the conditions that exclude the likelihood of their use:
- If the house has an open system.
- When using natural circulation in the circuits: such a concentrate of the coolant for heating the system simply "will not pull".
- The presence of pipes or other elements in contact with the coolant with a galvanized surface is unacceptable.
- All connecting assemblies equipped with seals made of tow or oil paint must be repacked, since glycolic substances will destroy them very quickly. As a result, antifreeze will start to leak, creating a real threat to people in the room. The old tow can be used as a new sealing material by treating it with a special sealing paste "Unipak"
- It is forbidden to use non-freezing liquids in those systems that are not equipped with devices for accurately maintaining the temperature of the coolant. The heating level dangerous for glycol antifreezes starts already from + 70-75 degrees: these processes are irreversible and fraught with the most unpleasant consequences.
- Usually, after pouring antifreeze into the system, it is required to increase the power of the pumping equipment, install a larger expansion tank, and increase the number of battery sections. Sometimes it is necessary to change pipes to wider ones.
- Incorrect operation of automatic air vents after pouring antifreeze was noticed: they are recommended to be replaced with Mayevsky taps.
- Before pouring antifreeze, the system must be thoroughly cleaned and rinsed. This is done using special formulations.
- To change the concentration level of antifreeze, use only distilled water. In this case, it is better to resist even from the use of purified and softened water.
- The correct concentration of antifreeze coolant for heating systems is of utmost importance. It is better not to expect that the winter will not be very harsh by overly diluting antifreeze. It is recommended to adhere to a threshold of -30 degrees, even in traditionally warm regions. In addition to protection against abnormal frosts, this will create optimal conditions for inhibitors and surfactants, the effectiveness of which is noticeably reduced with an excessive water content.
- After filling with new coolant, it is forbidden to immediately turn on the maximum mode of the system. It is best to build up power smoothly so that the antifreeze has time to adapt to new conditions and circuit elements.
- Studies show that at present the most reliable antifreeze coolant is a propylene glycol composition. Ethylene glycol is too dangerous, and glycerin is so controversial that it is rarely used. So it's better to overpay, but sleep well at night.
Content


Is it possible to pour an anti-freeze solution instead of antifreeze? The answer to this question should begin with a consideration of the composition of the liquid for glasses, as well as antifreeze. This will allow you to understand the principle of operation of these technical fluids, as well as the difference between them.
The basis of antifreeze is ethylene glycol
, or another higher alcohol. Also, various additives are added to antifreeze that prevent oxidation and destruction of parts of the power unit and the cooling system. In addition, there are substances that increase the boiling point of the liquid. Water is present in a small amount. The exact composition and proportions are usually not disclosed by the manufacturer, but a simple listing is enough for us.
Non-freezing is made from water diluted with isopropyl or methyl alcohol. Soap additives are also added there, which make it possible to more effectively clean the windshield from dirt. Antifreeze liquid for washers has such a simple and understandable composition.


What happens if you pour antifreeze into the washer reservoir?
YES absolutely nothing! It's completely safe! You will also wash your windshield with it, that's all!
Many can now say that ethylene glycol is much more toxic (antifreeze) than, say, methyl alcohol (non-freezing). YES, no guys, in terms of toxicity they are about the same, just glycols are much more expensive, about 2 - 3 times, than methyl alcohol, so it is simply inappropriate to use it in a “washing machine”!
For the tank itself, for nozzles and other washer systems, there will also be nothing wrong, on the contrary, they will be covered with a small layer of additives that should protect against corrosion, even if there is little metal there, if only in the pump, but in the nozzles, but at least them. Although such protection will quickly be washed off with subsequent anti-freeze! So do not panic, just ride and calmly throw out all the antifreeze or antifreeze.
So what can you pour antifreeze into a tank? YES, of course you can - only it costs 5 times more than the "omyvayka". For example, 1 liter of the cheapest antifreeze is about 60 - 80 rubles, but for 90 rubles you will buy 5 liters of anti-freeze!
Now the video version.
Did you like the article? Subscribe to the channel to keep abreast of the most interesting materials
Non-freezing in the radiator
To better understand why this liquid cannot be added to the radiator, let's look at an example of what consequences await the motor and the driver when using anti-freeze:
- This liquid contains a large amount of water. Accordingly, the processes will be significantly accelerated oxidation and corrosion
in the motor cooling jacket. This will bring the need to repair the power unit closer; - The alcohols used in antifreeze evaporate at temperatures from 65 ° C
(methyl alcohol) up to 8
2 ° C
(isopropyl alcohol). That is, at the operating temperature of the engine, the alcohol will evaporate from the liquid. This will create increased pressure in the system. The result may be
swollen conservator
, or a ripped off radiator cap. Also, do not forget that when the stove is on, vapors can get into the salon. This will cause poisoning; - If you do not get poisoned during the trip, and the radiator cap of your car will not go on a short flight, then you should not relax. Many adventures lie ahead. As a rule, alcohol evaporates during even a short trip. As a result, water with a small admixture of soap suds remains in your radiator. As you know, freezing of pure H2O occurs already at 0 ° C
... In practice, the engine cools down slowly, water freezing in the radiator occurs at a temperature of minus
3-5 ° C
... That is, even with a slight frost, you run the risk of freezing the cooling system. This will result in a complete overhaul of the system, in the worst case, an engine replacement will be required; - But, in the case when you are lucky, and no described negative phenomena have occurred, you will still have problems with the motor in the near future. It's all about soapy water. The pump whips it into foam, which clogs the channels and forms air pockets. As a result, the normal cooling of the motor is disrupted. Even if you drain the liquid and pour in normal antifreeze, the problem will not go anywhere. You will need to flush the engine, as well as remove plugs by blowing out the system, which is not so easy.
In addition, there is a risk of damage to rubber gaskets, the substances that make up the anti-freeze devices are often aggressive to rubber and some types of plastic. Do not be surprised that after such a procedure, incomprehensible leaks from the radiator will appear.
One conclusion can be drawn from all this. Do not use anti-freeze as an alternative to antifreeze. Of course, if your task is not to ditch the engine. There are many alternatives.


Instead of a coolant, he filled in a "washer" - what will happen?


After several of our readers immediately approached us with a similar problem, we could not ignore it.What will happen to the cooling system, if instead of the "cooling" fill "washer"? Expect expensive repairs or can you get by with a little blood - a complete replacement of the fluid? What is the threat of such an oversight on the road?
The case under consideration, for all its implausibility and even anecdotalism, is in reality by no means an isolated one, which is easy to verify by asking the appropriate question in any Internet search system.


And taking into account that it was possible to buy windscreen washer fluids with excise stamps, assuring that there is antifreeze in the container, it can be assumed that the reason for confusion where it is necessary to pour the "washer", and where - the coolant, may be not only absent-mindedness. But what is the risk of windscreen washer fluid entering the cooling system? The answer to this question should be prompted by the compositions of antifreezes and "washers".


Various non-freezing substances have been tried for the role of antifreeze, but aqueous solutions of ethylene glycol or propylene glycol meet the best requirements for fluids used in cooling systems. At what temperature glycol antifreezes freeze and boil depends on the ratio of the base components. For example, in antifreezes with a freezing point of -40 ° C, water and ethylene glycol or propylene glycol are mixed in a 50:50 ratio. What is also of great importance - the boiling point of such a mixture is 108 ° C. This allows antifreeze to cope better with clean water with temperature overloads and perform the functions of a coolant in the warm season of operation, when low-temperature properties remain unclaimed. At the same time, in its pure form, ethylene glycol boils at all at 197.3 ° C.


Glass-washing liquids are also aqueous solutions, but their second base component after water is ethyl, isopropyl or methyl alcohols. The main feature of these alcohols is that they boil at significantly lower temperatures than the liquid in the cooling system heats up during engine operation. In particular, methyl alcohol boils at 64.7 ° C, ethyl alcohol at 78.4 ° C, and isopropyl alcohol at 82.4 ° C. This alone makes these alcohols unsuitable for use in the cooling system, because it threatens the formation of steam locks and subsequent overheating of the engine. In addition, surfactants - surfactants designed to dissolve oily and soot deposits that appear on the windshield - are added to the "washer". With active stirring, the surfactants form a foam, the presence of which in the cooling system is also undesirable.
However, in our case, everything depends on the amount of "washer" mistakenly poured into the cooling system. The model of the car is not indicated, but even if we are talking about a subcompact engine, then its cooling system, as a rule, contains about five liters of antifreeze. Three hundred grams is just over 5% of the total coolant volume. It is unlikely that such a drop is capable of muddying the whole sea, so any serious negative and quick consequences seem unlikely.


To reduce their likelihood even more, you can not tightly close the cap of the expansion tank - alcohol, as well as perfume and substances that cause an emetic effect, which are added to "washers" so that alcohol lovers do not try to use them, evaporate faster. Surfactants will remain with their incomprehensible effect on the pump oil seal and other seals, but at least these substances do not have a negative effect on the rubber bands of the "wipers". What is still in question is the foam and how the additives contained in the antifreeze reacted to the appearance in the cooling system of the "washer" with its specific additives. If these issues look significant, then nothing prevents you from replacing the coolant, especially since it is still recommended to change it periodically.

By the way, about the expansion tank plug.It is equipped with a safety valve that opens when the pressure in the cooling system rises above normal. If this valve is faulty, then in the absence of leaks from the cooling system, it is he who can cause a decrease in the level of antifreeze due to the evaporation of water from it.
What to do if there is a leak?
So, having considered the features of the use of antifreeze liquid in the radiator, we turn to the question of what to do if a liquid leak is found along the way. Experienced motorists keep a small supply of coolant in the trunk for such a case. A liter bottle is enough. Please note that some types of modern antifreeze are not recommended to be mixed. Therefore, check in advance which variety you are using. This is especially important for new cars. With such a reserve, there will be no problems with a lack of coolant, you can always make up for the deficiency. But, do not forget to identify the cause of the leak, it is good if it is evaporation, worse if it is depressurization.
Do not forget to drain the mixture of water and antifreeze after arriving home. The freezing point of such a solution is lower, which can lead to defrosting of the system. Be sure to flush the system to remove salt deposits. After that, antifreeze is poured. It will not be possible to completely drain the water; there are several different methods for this.
Conclusion
... Almost every car enthusiast was faced with a lack of coolant in the car's radiator. In this regard, the question often arises whether it is possible to fill in an anti-freeze instead of antifreeze. In practice, this is strictly prohibited. Using this option is likely to result in serious engine problems.
Characteristics of antifreeze heating fluids
The way a low-freezing liquid for heating systems behaves in the circuit is primarily influenced by the quality of the additive package and, of course, the operating conditions. Regardless of which main active element is added to the glycol base, all formulations have anti-corrosion and anti-foaming properties.
Without these additives, the heating fluid is very corrosive. All non-freezing liquids foam, but especially glycerin anti-freezing liquids for heating systems of houses. Foam is an air-containing substance, and air leads to impaired circulation, the formation of air pockets, as well as water hammer in the heating system.
The additive package has its own time resource. After a certain time, the additives disintegrate at the molecular level.
This will form a precipitate and release acid. It turns out that nothing already smoothes the aggressiveness of the coolant for heating the house, moreover, everything is aggravated by the release of acid. Service life of antifreeze liquid:
- based on ethylene glycol - five years;
- based on propylene glycol - five years;
- glycerin-based - up to ten years.
This is the service life of the compound under favorable operating conditions. The main requirement is, of course, temperature. When the temperature of the coolant rises to 90 degrees, the non-freezing liquid begins to disintegrate and loses its properties. This only happens if the boiler starts up incorrectly after a long period of inactivity, or errors during installation.
Direct contact of the heat exchanger with the flame is undesirable if antifreeze is poured into the circuit
For example, when a heat exchanger is built into a conventional oven. Some people install it so that it is in contact with an open flame. If you plan to use antifreeze for stove heating, then this should not be done. It is necessary that there is a layer of brick between the heat exchanger and the flame. He and the coolant will protect from too hot tongues of flame, and distribute the heat evenly. In this case, the non-freezing liquid for stove heating will not overheat.
Characteristics influenced by the quality of the additive package:
- thermal conductivity;
- density;
- viscosity;
- fluidity;
- thermal expansion.
The higher the quality of the additives, the higher the characteristics will be. That is, as close as possible to the characteristics of water. In the case of the coefficient of thermal expansion, then it should be as small as possible.
Considering the fact that the volumetric expansion of the anti-freeze is greater than that of water, it is necessary to provide for an expansomat of 40% more volume.
The thermal conductivity of antifreeze is lower than that of water. The lowest thermal conductivity of glycerin antifreeze liquids. In relation to water, it is only 85%; in other non-freezing systems, the indicator can reach 90%. As you can see, the difference is not that great.
Non-freezing liquids are half as dense and viscous as water. These qualities impede circulation. In order to pump the coolant along the circuit, a pump of greater power will be required; it would also be nice to assemble a heating circuit from pipes with a cross section larger by one step. For example, when it comes to polypropylene pipes. then instead of 25 in diameter, it is better to take 32.
Despite the fact that the non-freezing liquid is denser and more viscous, it has a lower coefficient of surface tension, that is, it is more fluid. Do you know that you can draw water into a glass "with a slide"? The slide, of course, will be small, but even visually it is visible that the liquid rises above the edge of the vessel. With anti-freeze, this will not work. Due to this high fluidity, it flows out where water does not penetrate due to surface tension. In other words, if there are microcracks and even very small holes, then the non-freezing liquid will find a way out there.
Therefore, often, after there was water in the circuit and it was decided to pour an anti-freeze into it, leaks appear. Major leak points:
- pipe joints;
- connections between radiator sections;
- places for connecting additional elements;
- in the boiler itself.
Water has another useful property, thanks to which a minor leak can disappear by itself. Metal particles settle at the edges of the cracks and seal them. Of course, this is just scale, which, in the case of flushing and further pressure testing of the system, will be removed and flow will resume.
Is it possible to pour an anti-freeze solution instead of antifreeze? a question the answer
Hello, kindest uazovody! On the first cold morning, I noticed an interesting phenomenon in my yard. Semerovod, with whom I know "hello" poured an anti-freeze for glass into the radiator. At first I thought that the man simply did not have other canisters, so he poured antifreeze / antifreeze into such, and now he is putting it in place. It turned out not, he whips exactly the anti-freeze there and assures that this is an ancient and proven way to please the cooling system in winter. Besides, the method is cheap.
I must admit that I did not compare the prices of non-freezing and antifreeze, but there are some specific doubts about me. Is this really how they do it, or did the guy overpower the idiot? And it is Che, though cheaper.
I must admit that I did not compare the prices of non-freezing and antifreeze, but there are some specific doubts about me. Is this really how they do it, or did the guy overpower the idiot? And it is Che, though cheaper. 5L canister of normal domestic antifreeze - 200-300r, 4L of Castrolovsky concentrate - in the summer it cost about 400r. 5L bottle of anti-freeze - Rs 70-100 like.
As for "a fool or so they do" - IMHO a fool, but in Zhipul it will probably give a ride, and the Oise can generally go to the CO with anything, even with milk whey - it will also say thanks for it