Why connect batteries
A battery, like a capacitor, can store energy. Unlike a simple galvanic battery, where the chemical reactions that generate electricity are irreversible, the battery can be charged. In doing so, the ions are divorced from each other, and the internal chemistry of the battery is charged like a spring. Subsequently, these ions, due to the "charged" chemical process, will donate their extra electrons to the electrical circuit, themselves striving back to the neutrality of the acidic electrolyte.
All is well, only the battery has the amount of energy that it is able to generate after a full charge depends on its total mass. And the mass depends on the performance - there are standards, and batteries are made according to these standards. It is good when electricity consumption is similarly standardized. For example, when you have a car that takes a certain amount of electricity to start the engine. Well, for their other needs - feeding the automatics in the parking lot, powering locks with anti-theft devices, etc. Battery standards and are designed to power various types of vehicles.
And in other areas where a stable constant voltage is required, the demand for power parameters is much broader and more varied. Therefore, having the same type and strictly identical batteries, you can think about using them in different combinations, and more efficient charging methods than it is banal to charge them all in turn.
Connecting power supplies
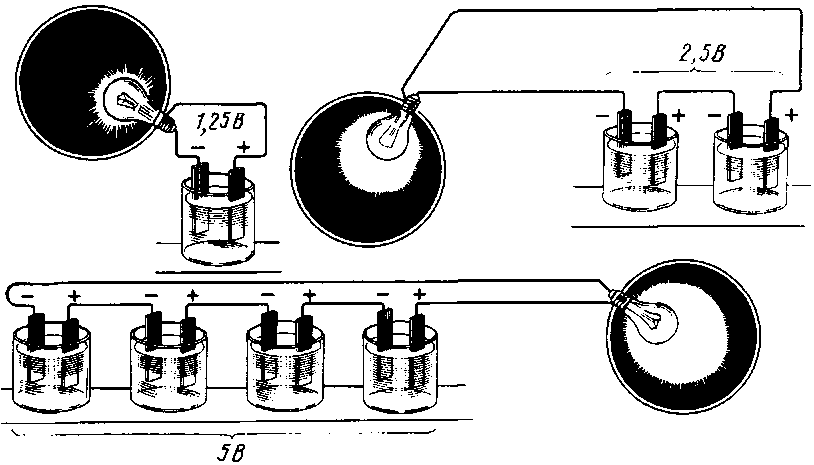
Like loads, for example, light bulbs, batteries can be connected both in parallel and in series.
At the same time, as one can immediately suspect, something must be summed up. When the resistors are connected in series, their resistance is summed up, the current on them will decrease, but through each of them it will go the same. Likewise, the current will flow the same through the serial connection of the batteries. And since there are more of them, the voltage at the battery outputs will increase. Consequently, with a constant load, a greater current will flow, which will use up the capacity of the entire battery in the same time as the capacity of one battery connected to this load.
Parallel connection of loads leads to an increase in the total current, while the voltage across each of the resistances will be the same. The same is with batteries: the voltage on a parallel connection will be the same as that of one source, and the current can all together give more. Or, if the load remains what it was, they will be able to supply it with current for as long as their total capacity has increased.
Now, having established that it is possible to connect the batteries in parallel and in series, we will consider in more detail how this works.
One-pipe connection of heating radiators
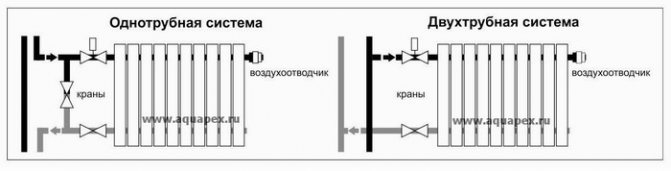
One-pipe radiator connection diagram is the simplest. The coolant is supplied and removed into the same pipe. But the ease of installation is decompensated by the shortcomings of such a system - all radiators in the network heat up unevenly, the first of them receives more heat, the last less. The temperature difference on the radiators of different ends of the network can be quite noticeable and reach ten degrees.
For this reason, one-pipe connection of heating radiators is best used on cast-iron batteries. When installing aluminum or bimetallic radiators, the temperature difference increases.
The lack of the system can be partially corrected by installing a bypass, which transfers the coolant from the upper supply pipe to the lower outlet pipe. A valve or thermostat is placed between the radiator inlet and the bypass for automation control.
How a chemical power supply works
Food sources based on chemical processes are primary and secondary. Primary sources consist of solid electrodes and electrolytes that connect them chemically and electrically - liquid or solid compounds. The complex of reactions of the entire unit acts in such a way that the chemical imbalance inherent in it is discharged, leading to a certain balance of components. The energy released in this case in the form of charged particles goes out and creates an electric voltage at the terminals. As long as there is no outflow of charged particles outside, the electric field slows down the chemical reactions inside the source. When you connect the terminals of the source with some electrical load, current will run through the circuit, and chemical reactions will resume with renewed vigor, again supplying electrical voltage to the terminals. Thus, the voltage at the source remains unchanged, slowly decreasing, as long as chemical imbalance remains in it. This can be observed by a slow, gradual decrease in voltage across the terminals.
This is called the discharge of a chemical source of electricity. Initially, such a complex was found to react with two different metals (copper and zinc) and an acid. In this case, metals are destroyed in the process of discharging. But then they selected such components and their interaction such that if, after reducing the voltage at the terminals as a result of discharge, it is artificially maintained there, then an electric current will flow back through the source, and chemical reactions can reverse, again creating the previous nonequilibrium state in the complex.
Sources of the first type, in which components are irretrievably destroyed, are called primary, or galvanic cells, after the discoverer of such processes, Luigi Galvani. Sources of the second kind, which, under the action of an external voltage, are capable of reversing the entire mechanism of chemical reactions, and again return to a nonequilibrium state inside the source, are called sources of the second kind, or electric accumulators. From the word "accumulate" - to thicken, to collect. And their main feature, just described, is called charging.
However, with batteries, things are not so simple.
Several such chemical mechanisms have been found. With different substances involved in them. Therefore, there are several types of batteries. And they behave differently, charge and discharge. And in some cases, phenomena arise that are very well known to people who deal with them.
And practically everyone deals with them. Batteries, as autonomous energy sources, are used everywhere, in a wide variety of devices. From small wristwatches to vehicles of various sizes: cars, trolleybuses, diesel locomotives, motor ships.
Some features of batteries
The classic battery is an automotive lead-sulphate battery. It is produced in the form of accumulators connected in series into the battery. Its use and charging / discharging are well known. Dangerous factors in them are corrosive sulfuric acid, which has a concentration of 25-30%, and gases - hydrogen and oxygen - that are released when charging continues after it is chemically finished. A mixture of gases resulting from the dissociation of water is precisely the well-known explosive gas, where hydrogen is exactly twice as much as oxygen. Such a mixture explodes at any opportunity - a spark, a strong blow.
Batteries for modern equipment - mobile phones, computers - are made in a miniature design; chargers of various designs are produced for charging them. Many of them contain control circuits that allow you to track the end of the charging process or charge all the elements in a balanced way, that is, disconnecting those that have already been charged from the device.
Most of these batteries are quite safe and improper discharging / charging can only damage them ("memory effect").
This applies to all, except for batteries based on the metal Li - lithium. It is better not to experiment with them, but to charge only on chargers specially designed for it and work with them only according to the instructions.
The reason is that lithium is very active. It is the third element in the periodic table after hydrogen, a metal that is more active than sodium.
When working with lithium-ion and other batteries based on it, lithium metal can gradually fall out of the electrolyte and once make a short circuit inside the cell. From this it can catch fire, which will lead to disaster. Since it CANNOT be paid off. It burns without oxygen, when it reacts with water. In this case, a large amount of heat is released, and other substances join the combustion.
There are known incidents of fire in mobile phones with lithium-ion batteries.
However, engineering thought is moving forward, creating more and more new chargeable cells based on lithium: lithium-polymer, lithium-nanowire. Trying to overcome the disadvantages. And they are very good as batteries. But ... away from sin it is better not to do with them those simple actions that are described below.
Two-pipe connection of heating radiators

Two-pipe systems have two pipelines in their design - direct and return. The cooled water from the radiator is returned to the boiler through the outlet pipe. Such a heating system is very convenient in that it allows you to ensure uniform heating of all radiators in the network and regulate their power separately.
Two-pipe systems can be horizontal or vertical. In the horizontal, the connection is carried out with top or bottom wiring. Vertical systems are convenient in houses with variable number of storeys.
Today, the two-pipe connection of heating radiators is considered more progressive and contributes to an increase in the comfort of living for people. In addition, they provide a more modern interior design and are convenient for concealed gaskets.
Serial connection of sources
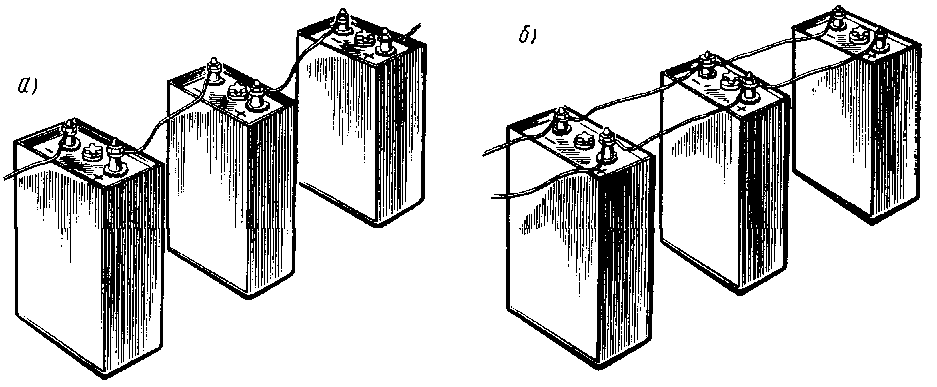
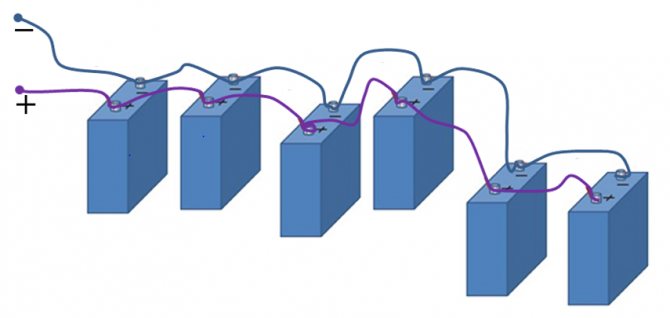
This is a well-known battery of cells, "cans". Consistently - this means that the plus of the first is brought out - there will be a positive terminal of the entire battery, and the minus is connected to the plus of the second. The minus of the second is with the plus of the third. And so on to the last. The minus of the penultimate one is connected to its plus, and its minus is brought out - the second terminal of the battery.
When the batteries are connected in series, the voltage of all the cells is added, and at the output - the plus and minus terminals of the battery - the sum of the voltages will be obtained.
For example, a car battery, having about 2.14 volts in each charged bank, gives a total of 12.84 volts out of six cans. 12 such cans (battery for diesel engines) will give 24 volts.
And the capacity of such a compound remains equal to the capacity of one can. As the output voltage is higher, the rated power of the load will increase and the power consumption will be faster. That is, everyone will be discharged at once together as one element.
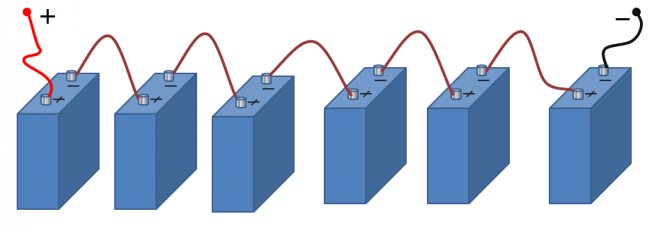

Series connection of batteries
These batteries are also charged in series. The plus of the supply voltage is connected to the plus, the minus to the minus.For normal charging, it is necessary that all banks are the same in parameters, from the same batch and equally discharged in unison.
Otherwise, if they are discharged slightly differently, then when charging, one will finish charging before the others and he will start recharging. And that could end badly for him. The same will be observed with different capacities of the elements, which, strictly speaking, are the same.
The series connection of batteries was tried from the very beginning, almost simultaneously with the invention of electrochemical cells. Alessandro Volta created his famous voltaic pillar from circles of two metals - copper and zinc, which he moved with cloths soaked in acid. The construction turned out to be a successful invention, practical, and even gave a voltage that was quite sufficient for the then daring experiments in the study of electricity - it reached 120 V - and became a reliable source of energy.
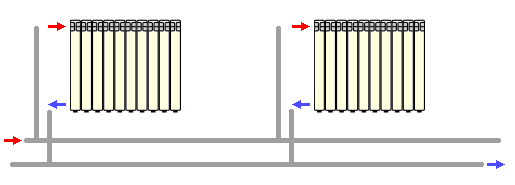
Diagonal connection of heating radiators

Diagonal connection of batteries with a heat supply line
Diagonal connection of radiators is the most effective option for the functioning of the heating system. With such a connection, the supply of hot coolant is carried out through the upper pipe on one side of the battery, and the return of chilled water to the riser is through the lower pipe on the other side. Such a connection provides the maximum level of heat transfer from the radiator and is recommended for use in relation to multi-section structures.
The imperfection of the diagonal connection of heating radiators is in its unattractive design. The appearance of an additional heating pipe around the radiator does not look very aesthetically pleasing, especially in the interior of office and presentation rooms. Most often, this type of connection is implemented in private housing construction, where great importance is attached to increasing the efficiency of the heating system, and design issues are given a secondary role.
Parallel connection of batteries
With a parallel connection of power supplies, all the pluses must be connected to one, creating a positive pole of the battery, all the minuses to the other, creating a minus of the battery.
Battery part

Parallel connection
With such a connection, the voltage, as we can see, should be the same on all elements. But what is it? If the batteries have different voltages before connection, then immediately after connection, the process of "equalization" will immediately begin. Those elements with a lower voltage will begin to recharge very intensively, drawing energy from those with a higher voltage. And it's good if the difference in voltages is explained by the different degree of discharge of the same elements. But if they are different, with different voltage ratings, then a recharge will begin, with all the ensuing charms: heating of the charged element, boiling of the electrolyte, loss of the metal of the electrodes, and so on. Therefore, before connecting the elements to each other in a parallel battery, it is necessary to measure the voltage on each of them with a voltmeter to make sure that the upcoming operation is safe.
As we can see, both methods are quite viable - both parallel and serial connection of batteries. In everyday life, we have enough of those elements that are included in our gadgets or cameras: one battery, or two, or four. They are connected the way it is defined by the design, and we do not even think about whether this is a parallel or serial connection.
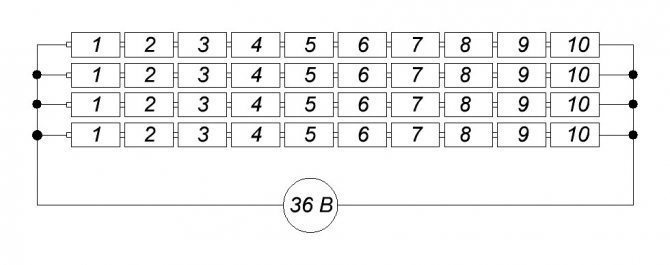
But when in technical practice it is necessary to immediately provide a large voltage, and even for a long period, huge fields of accumulators are built in the premises.
For example, for emergency power supply of a radio relay communication station with a voltage of 220 volts during the period when any failure in the power circuit must be eliminated, it takes 3 hours ... There are a lot of batteries.
Similar articles:
- Methods for converting 220 volts to 380
- Calculation of voltage losses in the cable
- Working with a megohmmeter: what is it for and how to use it?
Bottom connection of heating radiators


Bottom radiator connection
Such a scheme for connecting heating radiators is considered the least efficient in terms of heat transfer. The thermal power of radiators when using it is significantly reduced, and heat loss reaches 10-15%. For this reason, the use of radiators with bottom connection is avoided. But in cases where the aesthetic side of the issue is assigned an important role in the interior of the premises, for example, in the premises of company offices, such a scheme is very convenient. Either when installing designer radiators with complex shapes or non-standard placement. It effectively hides pipelines, which are most often masked with baseboards or embedded in the floor screed.
Such a piping is justified when using bimetallic or aluminum radiators, in which the high thermal conductivity of the material of manufacture helps to reduce heat transfer losses.