Chemical stability
Considering the chemical properties of gasoline, the main emphasis should be placed on how long the composition of hydrocarbons will remain unchanged, since with long storage, lighter components disappear and performance is greatly reduced.
In particular, the problem is acute if a higher grade fuel (AI 95) was obtained from gasoline with a minimum octane number by adding propane or methane to its composition. Their anti-knock properties are higher than that of isooctane, but they also dissipate instantly.
According to GOST, the chemical composition of fuel of any brand must be unchanged for 5 years, subject to storage rules. But in fact, often even the newly purchased fuel already has an octane number below the specified one.
Unscrupulous sellers are to blame for this, who add liquefied gas to containers with fuel, the storage time of which has expired, and the content does not meet the requirements of GOST. Usually, different amounts of gas are added to the same fuel to obtain an octane number of 92 or 95. Confirmation of such tricks is the pungent smell of gas at the filling station.
Speed - Combustion - Fuel
What is the real cost of 1 liter of gasoline
The fuel combustion rate increases greatly if the combustible mixture is in intense vortex (turbulent) motion. Accordingly, the intensity of turbulent heat transfer can be much higher than that of molecular diffusion.
The combustion rate of fuel depends on a number of reasons discussed later in this chapter and, in particular, on the quality of mixing of fuel with air. The rate of fuel combustion is determined by the amount of fuel burned per unit of time.
The fuel combustion rate and, consequently, the heat release rate are determined by the size of the combustion surface. Coal dust with a maximum particle size of 300 - 500 microns has a combustion surface tens of thousands of times larger than coarse sorted chain grate fuel.
The rate of fuel combustion depends on the temperature and pressure in the combustion chamber, increasing with their increase. Therefore, after ignition, the combustion rate increases and becomes very high at the end of the combustion chamber.
The speed of fuel combustion is also influenced by the engine speed. With an increase in the number of revolutions, the duration of the phase is reduced.
The turbulence of the gas flow sharply increases the rate of fuel combustion due to an increase in the area of the combustion surface and the speed of propagation of the flame front with an increase in the rate of heat transfer.
When operating on a lean mixture, the combustion rate of the fuel is slowed down. Therefore, the amount of heat given off by gases to parts increases and the engine overheats. Signs of a lean mixture are flashes in the carburetor and intake manifold.
The turbulence of the gas flow sharply increases the rate of fuel combustion due to an increase in the combustion surface area and the speed of propagation of the flame front due to an increase in the rate of heat transfer.
Normal alkanes have the maximum cetane number, which characterizes the rate of fuel combustion in an engine.
The composition of the working mixture greatly affects the rate of combustion of fuel in the engine. These conditions take place at coeff.
The influence of the quality of the development of the combustion process is determined by the rate of fuel combustion in the main phase. When a large amount of fuel is burned in this phase, the values of pz and Tz increase, the proportion of after-burning fuel decreases during the expansion process, and the polytrope index nz becomes larger.This development of the process is the most favorable, since the best heat utilization is achieved.
In the working process of the engine, the value of the rate of fuel combustion is very important. The combustion rate is understood as the amount (mass) of fuel reacting (burning) per unit of time.
A number of general phenomena indicate that the rate of fuel combustion in engines is quite natural, not random. This is indicated by the reproducibility of more or less unambiguous cycles in the engine cylinder, which, in fact, determines the stable operation of the engines. In the same engines, the protracted nature of combustion is always observed with lean mixtures. Hard work of the engine, which occurs at a high speed of combustion reactions, is observed, as a rule, in compressorless diesel engines, and soft work - in engines with ignition from an electric spark. This indicates that fundamentally different mixture formation and ignition cause a regular change in the combustion rate. With an increase in the number of engine revolutions, the duration of combustion decreases in time, and in the angle of rotation of the crankshaft, it increases. The kinetic curves of the course of burnup in engines are similar in nature to the kinetic curves of a number of chemical reactions that are not directly related to engines and occurring under different conditions.
Experiments indicate the dependence of the intensity of radiant heat transfer on the rate of fuel combustion. With rapid combustion at the root of the torch, higher temperatures develop and heat transfer intensifies. The inhomogeneity of the temperature field, along with different concentrations of emitting particles, leads to inhomogeneity of the degree of flame blackness. All of the above creates great difficulties for the analytical determination of the temperature of the radiator and the degree of emissivity of the furnace.
With a laminar flame (see Section 3 for more details), the fuel combustion rate is constant and Q 0; the combustion process is silent. However, if the combustion zone is turbulent, and this is the case under consideration, then even if the fuel consumption is constant on average, the local combustion rate changes in time and for a small volume element Q.Q. Turbulence is continually disturbing the flame; at any given moment, the combustion is limited by this flame or a series of flames occupying a random position in the combustion zone.
Combustion temperature and calorific value of firewood
Probably everyone was faced with the problem of kindling a fire at their summer cottage or firewood in the grill / fireplace at home, and asked himself the question - why they do not light up. So, as a rule, the logs do not light up, tk. conditions have not been created for their kindling, namely, there is no temperature.
After all, not everyone knows that in order to light firewood, a temperature of more than 290-320 degrees Celsius is needed for almost any type of wood. At the same time, the tree itself burns at a temperature of about 850-950 degrees. In this case, for example, ordinary coal ignites at a temperature of 550-650 degrees, and the combustion temperature is from 1000 to 1300 degrees Celsius.
And how to determine what is the temperature in a fire, fireplace or barbecue with your own hands without improvised means?
You can simply find out the temperature at which wooden logs are burning - by the color of burning wooden firewood, because the color of the wood changes depending on the temperature at which they burn under the influence of combustion and oxidation products.
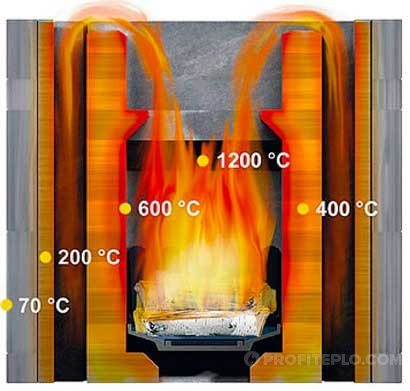
Almost everyone loves to watch the flames. The main function of a fire is to heat the room and heat various objects. Private homes use solid fuels. It must be understood that the combustion temperature of firewood in any stove depends on the stove structure, conditions, and also on the type of wood. Therefore, different logs perform specific tasks.
In order for the material or propane to start burning in the furnace, it needs oxygen.The interaction of organic material with oxygen during combustion gives off carbon dioxide and water vapor, which is expelled through a specially installed chimney in the furnace structure.
Any combustible fuel has a specific chemical composition. The internal composition of wood, oil or coal also differs. For example, coal can contain a small or significant amount of ash. Wood can give off different temperatures and also has an excellent food composition.
The combustion temperature is checked in special laboratories using a comparative test, since it is simply impossible to carry out this procedure at home on your own. To obtain accurate results, the wood must be dried to a specified moisture content.
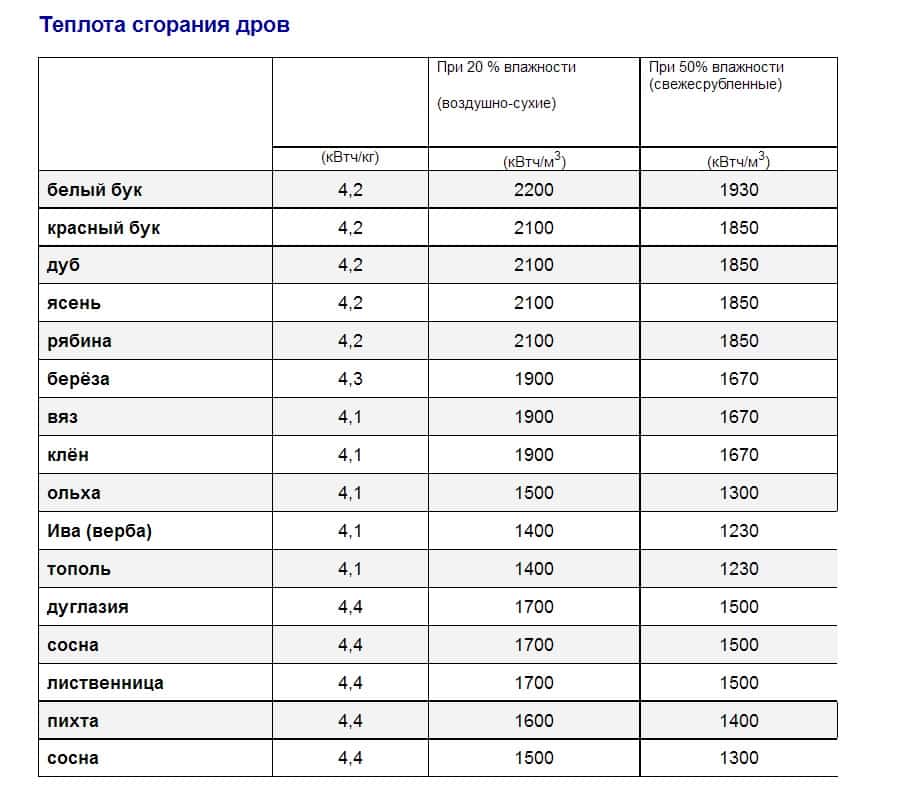
Thermal capacity of wood:
- Birch - 4968.
- Pine 4907-4952.
- Spruce - 4860.
- Alder - 5050.
- Aspen - 4950.
Before using firewood, it is necessary to take into account the degree of dryness, because wet fuel will burn poorly, as a result of which it emits a minimum of heat. Therefore, before using solid fuel in a wood-burning stove, it must be kept in a dry room for a while to dry it out.
It is important to note that the burning temperature of wood is an imprecise concept. Combustible materials should be evaluated for their ability to generate some heat. This indicator is measured in calories (a unit of heat required to heat water by one degree).
Firewood quality
The thermal conductivity of wood in the stove depends on the moisture content in it. Any tree contains a large amount of water, which is extracted by the roots. During combustion, such fuel will emit not only heat, but also steam, as the water evaporates.
To better understand this, you need to know that if the wood contains no more than 15% water, then its heat output will be approximately 3660 calories. Compared to dry fuel, this is a very low figure.
Using raw fuel is tantamount to just throwing away some of the dry fuel. Moisture reduces heat transfer so much that it would be enough to heat ten liters of water.
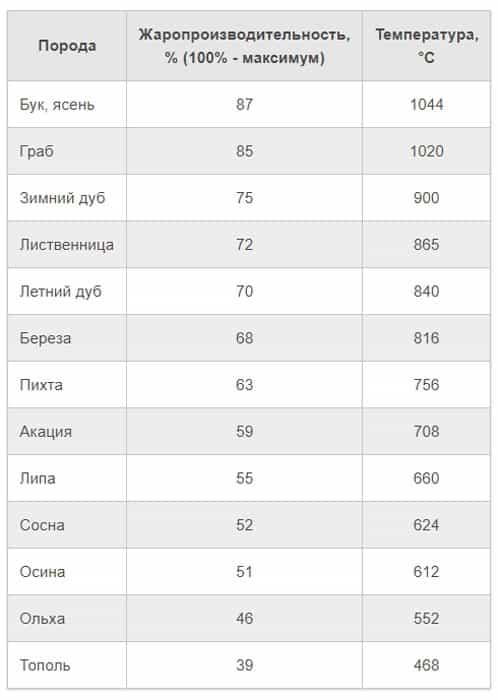
Most often, people use firewood from hornbeam, beech, pine, oak, birch and acacia. Pine harvested in summer, larch, maple and ash give the most heat. Also, preference should be given to oak, which is felled in the summer, its temperature allows you to heat a large room.
Chestnut, cedar, fir and spruce give off less heat. It is not recommended to prepare fuel from poplar, aspen, alder, willow and linden, as they contain a large amount of moisture.
It is best to harvest wood for the stove from heavy and dense wood.
Any firewood burns the same way: some are almost completely, others have some kind of remnants. It depends not only on the chemical reaction and the type of fuel, but also on the furnace itself. For heating, you should choose firewood, the heat transfer of which is at least 3800 calories.
A traditional thermometer is not suitable for measuring fuel temperature. This procedure requires a special device called a pyrometer.
It is important to note that a high combustion temperature is not an indication that the wood will have a high heat transfer. Much depends on the design of the oven. To increase the temperature, it is enough to reduce the amount of oxygen supplied.
Advice
- If the oven door is tightly closed, and at the same time smells of dampness, then you need to check the tightness of the structure.
- The chimney must withstand aggressive environments well, as the wood contains various acids.
- In the case of using wood containing resin, the chimney must be thoroughly cleaned.
- To quickly heat up the room, it is recommended to increase the oxygen supply and use firewood, the combustion temperature of which is higher than the rest.
To understand the process of heating a room using stove equipment, it is imperative to know about the combustion temperature of the fuel.
Firewood is a classic solid fuel option in forested areas. Burning wood makes it possible to obtain thermal energy, while the combustion temperature of the wood directly affects the efficiency of fuel use. The flame temperature depends on the type of wood, as well as on the moisture content of the fuel and the conditions of its combustion.
The combustion temperature of wood determines the heat transfer rates of the fuel - the higher it is, the more heat energy is released during the combustion of firewood. In this case, the specific heating value of the fuel depends on the characteristics of the wood.
Heat transfer indicators in the table are indicated for firewood burned under ideal conditions:
- minimum moisture content in the fuel;
- combustion takes place in a closed volume;
- oxygen supply is metered - the amount that is necessary for full combustion is supplied.
It makes sense to be guided by the tabular values of the calorific value only for comparing different types of firewood with each other - in real conditions, the heat transfer of the fuel will be noticeably lower.
What is combustion
Combustion is an isothermal phenomenon - that is, a reaction with the release of heat.
1. Warming up. The piece of wood must be heated with an external fire source to the ignition temperature. When heated to 120-150 degrees, the wood begins to char, and coal is formed, capable of spontaneous combustion. When heated to 250-350 degrees, the process of thermal decomposition into gaseous components (pyrolysis) starts.
2. Combustion of pyrolysis gases. Further heating leads to increased thermal decomposition, and the concentrated pyrolysis gases flare up. After the outbreak, the ignition gradually begins to cover the entire heating zone. This produces a stable light yellow flame.
3. Ignition. Further heating will ignite the wood. The ignition temperature in natural conditions ranges from 450 to 620 degrees. The wood ignites under the influence of an external source of thermal energy, which provides the heating necessary for a sharp acceleration of the thermochemical reaction.
The flammability of wood fuel depends on a number of factors:
- volumetric weight, shape and section of a wood element;
- the degree of moisture in the wood;
- traction force;
- the location of the object to be ignited relative to the air flow (vertical or horizontal);
- density of wood (porous materials ignite more easily and faster than dense ones, for example, it is easier to light alder wood than oak).
For ignition, good, but not excessive traction is required - a sufficient supply of oxygen and a minimum dissipation of the thermal energy of combustion are required - it is needed to warm up adjacent sections of wood.
4. Combustion. Under conditions close to optimal, the initial outbreak of pyrolysis gases does not fade, from ignition the process turns into stable combustion with a gradual coverage of the entire volume of fuel. Combustion is divided into two phases - smoldering and flaming combustion.
Smoldering involves the combustion of coal, a solid product of the pyrolysis process. The release of flammable gases is slow and they do not ignite due to insufficient concentration. Gaseous substances, when cooled, condense, forming a characteristic white smoke. In the process of smoldering, air penetrates deep into the wood, due to which the coverage area expands. Flame combustion is provided by the combustion of pyrolysis gases, while the hot gases move outward.
Combustion is maintained as long as there are conditions for fire - the presence of unburned fuel, oxygen supply, maintaining the required temperature level.
5. Attenuation. If one of the conditions is not met, the combustion process stops and the flame goes out.
To find out what is the burning temperature of wood, use a special device called a pyrometer. Other types of thermometers are not suitable for this purpose.
There are recommendations to determine the combustion temperature of wood fuel by the color of the flame. Dark red flames indicate low-temperature combustion, white flames indicate high temperatures due to increased draft, in which most of the heat energy goes into the chimney. The optimal color of the flame is yellow, this is how dry birch burns.
In solid fuel boilers and stoves, as well as in closed fireplaces, it is possible to adjust the flow of air into the firebox by adjusting the intensity of the combustion process and heat transfer.
Boiling - gasoline
Octane number Gasoline composition
Gasoline starts boiling at a relatively low temperature and proceeds very intensively.
The end of the boiling point of gasoline is not specified.
The beginning of boiling of gasoline is below 40 C, the end is 180 C, the temperature of the beginning of crystallization is not higher than 60 C. The acidity of gasoline does not exceed 1 mg / 100 ml.
The end boiling point of gasoline according to GOST is 185 C, and the actual one is 180 C.
The end-boiling point of gasoline is the temperature at which a standard (100 ml) portion of the test gasoline is completely distilled (boiled away) from the glass flask in which it was located into the refrigerator-receiver.
| Stabilization installation diagram. |
The final boiling point of gasoline should not exceed 200 - 225 C. For aviation gasolines, the final boiling point is much lower, reaching in some cases up to 120 C.
MPa, the boiling point of gasoline is 338 K, its average molar mass is 120 kg / kmol, and the heat of vaporization is 252 kJ / kg.
The initial boiling point of gasoline, for example 40 for aviation gasoline, indicates the presence of light, low-boiling fractions, but does not indicate their content. The boiling point of the first 10% fraction, or starting temperature, characterizes the starting properties of gasoline, its volatility, as well as the tendency to form gas plugs in the gasoline supply system. The lower the boiling point of the 10% fraction, the easier it is to start the engine, but also the greater the possibility of the formation of gas locks, which can cause interruptions in the fuel supply and even stop the engine. Too high boiling point of the starting fraction makes it difficult to start the engine at low ambient temperatures, which leads to losses of gasoline.
| Influence of the end point of boiling point of gasoline on its consumption during vehicle operation. The effect of the distillation temperature of 90% gasoline on the octane number of gasolines of various origins. |
A decrease in the end of the boiling point of reforming gasolines leads to a deterioration in their detonation resistance. Research and economic calculations are needed to address this issue. It should be noted that in the foreign practice of a number of countries, motor gasolines with a boiling point of 215 - 220 C are currently being produced and used.
| Influence of the end point of boiling point of gasoline on its consumption during vehicle operation. Influence of the distillation temperature of 90% gasoline on the octane number of gasolines of various origins. |
A decrease in the end of the boiling point of reforming gasolines leads to a deterioration in their detonation resistance. Research and economic calculations are needed to address this issue. It should be noted that in the foreign practice of a number of countries, motor gasolines with a boiling point of 215 - 220 C are currently being produced and used.
If the end-boiling point of gasoline is high, then the heavy fractions contained in it may not evaporate, and, therefore, not burn out in the engine, which will lead to increased fuel consumption.
Lowering the end-boiling point of straight-run gasolines leads to an increase in their detonation resistance.Low-octane straight-run gasolines have octane numbers of 75 and 68, respectively, and are used as components of motor gasolines.
What is the combustion process

An isothermal reaction in which a certain amount of thermal energy is released is called combustion. This reaction goes through several successive stages.
In the first stage, the wood is heated by an external fire source to the point of ignition. As it heats up to 120-150 ℃, the wood turns into charcoal, which is capable of spontaneous combustion. Upon reaching a temperature of 250-350 ℃, flammable gases begin to evolve - this process is called pyrolysis. At the same time, the top layer of wood smolders, which is accompanied by white or brown smoke - these are mixed pyrolysis gases with water vapor.
At the second stage, as a result of heating, the pyrolysis gases light up with a light yellow flame. It gradually spreads to the entire area of the wood, continuing to heat the wood.
The next stage is characterized by the ignition of the wood. As a rule, for this, it must warm up to 450-620 ℃. In order for the wood to ignite, an external source of heat is needed, which will be intense enough to rapidly heat the wood and accelerate the reaction.
In addition, factors such as:
- traction;
- moisture content of wood;
- section and shape of firewood, as well as their number in one tab;
- wood structure - loose firewood ignites faster than dense wood;
- placement of the tree relative to the air flow - horizontally or vertically.
Let's clarify some points. Since damp wood, when burning, first of all evaporates excess liquid, it ignites and burns much worse than dry wood. Shape also matters - ribbed and serrated logs ignite more easily and faster than smooth and round ones.
The draft in the chimney must be sufficient to ensure the flow of oxygen and dissipate thermal energy inside the firebox to all objects in it, but not blow out the fire.


The fourth stage of the thermochemical reaction is a stable combustion process, which, after the outbreak of pyrolysis gases, covers all the fuel in the furnace. Combustion takes place in two phases - smoldering and burning with a flame.
In the process of smoldering, the coal formed as a result of pyrolysis burns, while the gases are released rather slowly and cannot ignite due to their low concentration. Condensing gases produce white smoke as they cool. When the wood smolders, fresh oxygen gradually penetrates inside, which leads to a further spread of the reaction to all other fuels. The flame arises from the combustion of pyrolysis gases, which move vertically towards the exit.
As long as the required temperature is maintained inside the furnace, oxygen is supplied and there is unburned fuel, the combustion process continues.
If these conditions are not maintained, then the thermochemical reaction passes into the final stage - attenuation.
Combustion - gasoline
Design and principle of operation Bosch Motronic MED 7 direct petrol injection system
Combustion of gasoline, kerosene and other liquid hydrocarbons occurs in the gas phase. Combustion can occur only when the concentration of fuel vapor in the air is within certain limits, individual for each substance. If a small amount of fuel vapors is contained in the IB air, then combustion will not occur, as well as in the case when there is too much fuel vapors and not enough oxygen.
| Temperature change on the surface of kerosene during extinguishing with foams. | Temperature distribution in kerosene before the start of extinguishing (a and at the end. |
When gasoline burns, as is known, a homothermal layer is formed, the thickness of which increases with time.
When gasoline burns, water and carbon dioxide are formed. Can this serve as sufficient confirmation that gasoline is not an element?
When gasoline, kerosene and other liquids are burned in tanks, the crushing of the gas flow into separate volumes and the combustion of each of them separately are especially clearly visible.
When gasoline and oil are burned in large-diameter tanks, the character of heating differs significantly from that described above. When they burn, a heated layer appears, the thickness of which naturally increases over time and the temperature is the same as the temperature on the surface of the liquid. Under it, the temperature of the liquid drops rapidly and becomes almost the same as the initial temperature. The nature of the curves shows that during combustion, gasoline breaks down into two layers - an upper and a lower one.
For example, burning gasoline in air is called a chemical process. In this case, energy is released, equal to approximately 1300 kcal per 1 mole of gasoline.
Analysis of the combustion products of gasoline and oils is becoming extremely important, since knowledge of the individual composition of such products is necessary for the study of combustion processes in the engine and for the study of air pollution.
Thus, when gasoline is burned in wide tanks, up to 40% of the heat released as a result of combustion is consumed for radiation.
Table 76 shows the burning rate of gasoline with tetranitro-methane additives.
Experiments have found that the speed of gasoline burning from the surface of the tank is significantly affected by its diameter.
| Alignment of forces and means when extinguishing a fire on the stretch. |
With the help of GPS-600, firefighters successfully coped with the elimination of the burning of gasoline that spilled along the railway track, ensuring the movement of the trunk operators to the place where the tanks were coupled. Having disconnected them, with a piece of a contact wire, they attached 2 tanks with gasoline to the fire engine and pulled them out of the fire zone.
| The rate of heating of oils in tanks of various diameters. |
A particularly large increase in the speed of warming up from the wind was noticed when burning gasoline. When gasoline was burning in a tank of 2 64 m at a wind speed of 1 3 m / s, the heating rate was 9 63 mm / min, and at a wind speed of 10 m / s, the heating rate increased to 17 1 mm / min.
Humidity and combustion intensity
If the wood was recently felled, then it contains from 45 to 65% moisture, depending on the season and species. With such raw firewood, the combustion temperature in the fireplace will be low, since a large amount of energy will be spent on the evaporation of water. Consequently, the heat transfer from raw wood will be quite low.
There are several ways to achieve the optimal temperature in the fireplace and release a sufficient amount of heat energy to warm up:
- Burn twice as much fuel at a time to heat the house or cook food. This approach is fraught with significant material costs and increased accumulation of soot and condensate on the walls of the chimney and in the passages.
- Raw logs are sawn, chopped into small logs and placed under a canopy to dry. As a rule, firewood loses up to 20% moisture in 1-1.5 years.
- Firewood can be purchased already well dried. Although they are somewhat more expensive, the heat transfer from them is much greater.
At the same time, raw birch firewood has a fairly high calorific value. In addition, raw logs from hornbeam, ash and other types of wood with dense wood are suitable for use.
Temperature - combustion - fuel
| Dependence of criterion B on the ratio of the area of heat sources to the area of the workshop. |
The intensity of the worker's irradiation depends on the combustion temperature of the fuel in the furnace, the size of the charging hole, the thickness of the furnace walls at the charging hole and, finally, on the distance at which the worker is from the charging hole.
| The CO / CO and H2 / HO ratios in the products of incomplete combustion of natural gas, depending on the air consumption coefficient a. |
The practically achievable temperature 1L is the combustion temperature of the fuel in real conditions. When determining its value, heat losses to the environment, the duration of the combustion process, the method of combustion and other factors are taken into account.
Excess air dramatically affects the combustion temperature of the fuel. So, for example, the actual combustion temperature of natural gas with a 10% excess of air is 1868 C, with a 20% excess of 1749 C and with a 100% excess of air, it decreases to 1167 C. On the other hand, the preheating of air, going to the combustion of fuel, increases the temperature of its combustion. So, when burning natural gas (1Max 2003 C) with air heated to 200 C, the combustion temperature rises to 2128 C, and when the air is heated to 400 C - up to 2257 C.
| General diagram of the furnace device. |
When air and gaseous fuel are heated, the combustion temperature of the fuel rises, and, consequently, the temperature of the working space of the furnace also rises. In many cases, reaching the temperatures required for a given technological process is impossible without high heating of air and gaseous fuel. For example, steel smelting in open-hearth furnaces, for which the temperature of the torch (flow of burning gases) in the melting space should be 1800 - 2000 C, would be impossible without heating air and gas to 1000 - 1200 C. When heating industrial furnaces low-calorie local fuel (damp firewood, peat, brown coal), their work without heating the air is often even impossible.
It can be seen from this formula that the combustion temperature of the fuel can be increased by increasing its numerator and decreasing the denominator. The dependence of the combustion temperature of various gases on the excess air ratio is shown in Fig.
Excess air also sharply affects the combustion temperature of the fuel. So, the heat output of natural gas with an excess of air of 10% - 1868 C, with an excess of air of 20% - 1749 C and with a 100% excess is equal to 1167 C.
If the hot junction temperature is limited only by the combustion temperature of the fuel, the use of recuperation makes it possible to increase the temperature Тт by increasing the temperature of the combustion products and thus increase the overall efficiency of the TEG.
The enrichment of the blast with oxygen leads to a significant increase in the combustion temperature of the fuel. As the graph data in Fig. 17, the theoretical temperature of fuel combustion is associated with the enrichment of the blast with oxygen by a dependence, which is practically linear up to the oxygen content in the blast of 40%. At higher degrees of enrichment, the dissociation of combustion products begins to have a significant effect, as a result of which the curves of the temperature dependence on the degree of enrichment of the blast deviate from straight lines and asymptotically approach the temperatures limiting for a given fuel. Thus, the considered dependence of the fuel combustion temperature on the degree of oxygen enrichment of the blast has two regions - a region of relatively low enrichments, where there is a linear dependence, and a region of high enrichments (over 40%), where the temperature rise has a decaying character.
An important thermotechnical indicator of the furnace operation is the furnace temperature, which depends on the combustion temperature of the fuel and the nature of heat consumption.
The ash of the fuel, depending on the composition of the mineral impurities, at the temperature of the combustion of the fuel can be fused into pieces of slag. The characteristic of fuel ash depending on temperature is given in table. BUT.
The value of tmaK in table. IV - З - calorimetric (theoretical) temperature of fuel combustion.
Heat losses through the walls of the furnaces to the outside (into the environment) reduce the combustion temperature of the fuel.
Combustion temperature of various types of coal
Wood species differ in density, structure, quantity and composition of resins. All these factors affect the calorific value of the wood, the temperature at which it burns, and the characteristics of the flame.
Poplar wood is porous, such firewood burns brightly, but the maximum temperature indicator reaches only 500 degrees. Dense wood species (beech, ash, hornbeam), when burned, emit over 1000 degrees of heat. Indicators of birch are slightly lower - about 800 degrees. Larch and oak flare up hotter, giving out up to 900 degrees Celsius. Pine and spruce firewood burns at 620-630 degrees.
Birch firewood has a better ratio of heat efficiency and cost - it is economically unprofitable to heat with more expensive woods with high combustion temperatures.
Spruce, fir and pine are suitable for making fires - these conifers provide relatively moderate warmth. But it is not recommended to use such firewood in a solid fuel boiler, in a stove or fireplace - they do not emit enough heat to effectively heat the home and cook food, burn out with the formation of a large amount of soot.


Low-quality firewood is considered to be fuel made from aspen, linden, poplar, willow and alder - porous wood emits little heat when burning. Alder and some other types of wood "shoot" with coals during combustion, which can lead to a fire if the wood is used to fire an open fireplace.
When choosing, you should also pay attention to the degree of moisture content of the wood - raw firewood burns worse and leaves more ash.
Depending on the structure and density of wood, as well as the amount and characteristics of resins, the combustion temperature of firewood, their calorific value, as well as the properties of the flame depend.
If the tree is porous, then it will burn very brightly and intensely, but it will not give high combustion temperatures - the maximum indicator is 500 ℃. But denser wood, such as hornbeam, ash or beech, burns at a temperature of about 1000 ℃. The burning temperature is slightly lower for birch (about 800 ℃), as well as oak and larch (900 ℃). If we are talking about such species as spruce and pine, then they light up at about 620-630 ℃.
When choosing a type of firewood, it is worth considering the ratio of the cost and heat capacity of a particular wood. As practice shows, the best option can be considered birch firewood, in which these indicators are best balanced. If you buy more expensive firewood, the costs will be less efficient.
For heating a house with a solid fuel boiler, it is not recommended to use such types of wood as spruce, pine or fir. The fact is that in this case, the combustion temperature of the wood in the boiler will not be high enough, and a lot of soot will accumulate on the chimneys.
Low heat efficiency values are also found in alder, aspen, linden and poplar firewood due to its porous structure. In addition, sometimes alder and some other types of firewood are shot with coals during the combustion process. In the case of an open furnace, such micro explosions can lead to fires.
In addition to the calorific value, that is, the amount of heat energy released during fuel combustion, there is also the concept of heat output. This is the maximum temperature in a wood-burning stove that a flame can reach at the time of intense wood burning. This indicator also completely depends on the characteristics of the wood.
In particular, if the wood has a loose and porous structure, it burns at rather low temperatures, forming a bright high flame, and gives quite little heat. But dense wood, although it flares up much worse, even with a weak and low flame gives high temperature and a large amount of thermal energy.
The efficiency and economy of a heating system with a solid fuel boiler directly depends on the type of fuel. In addition to firewood and woodworking waste, various types of coal are actively used as an energy source.The combustion temperature of coal is one of the important indicators, but should it be taken into account when choosing a fuel for a furnace or boiler?
Coals primarily differ in origin. Charcoal, which is obtained by burning wood, as well as fossil fuels are used as an energy carrier.
Fossil coals are natural fuels. They consist of the remains of ancient plants and bituminous masses, which have undergone a number of transformations in the process of sinking into the ground to great depths.
The transformation of the initial substances into effective fuel proceeded at high temperatures and under conditions of oxygen deficiency under the earth. Fossil fuels include lignite, bituminous coal and anthracite.
Brown coals
Among the fossil coals, the youngest are brown coals. The fuel got its name for its brown color. This type of fuel is characterized by a large amount of volatile impurities and a high moisture content - up to 40%. Moreover, the amount of pure carbon can reach 70%.
Due to the high humidity, brown coal has a low combustion temperature and low heat transfer. Fuel ignites at 250 ° C, and the combustion temperature of brown coal reaches 1900 ° C. The calorific value is approximately 3600 kcal / kg.
As an energy carrier, brown coal in its natural form is inferior to firewood, therefore it is rarely used for stoves and solid fuel units in private houses. But briquetted fuel is in steady demand.
Lignite in briquettes is a specially prepared fuel. By reducing humidity, its energy efficiency is increased. The heat transfer of briquetted fuel reaches 5000 kcal / kg.
Hard coals
Bituminous coals are older than brown coals, their deposits are located at a depth of up to 3 km. In this type of fuel, the content of pure carbon can reach 95%, and volatile impurities - up to 30%. This energy carrier contains no more than 12% moisture, which has a positive effect on the thermal efficiency of the mineral.
The combustion temperature of coal in ideal conditions reaches 2100 ° C, but in a heating furnace the fuel is burned at a maximum of 1000 ° C. Heat transfer of coal fuel is 7000 kcal / kg. It is more difficult to ignite - heating up to 400 ° C is required for ignition.
Coal energy is most often used for heating residential buildings and buildings for other purposes.
Anthracite
The oldest solid fossil fuel, which is practically free of moisture and volatile impurities. The carbon content in anthracite exceeds 95%.
Specific heat transfer of fuel reaches 8500 kcal / kg - this is the highest indicator among coals. Under ideal conditions, anthracite burns at 2250 ° C. It ignites at a temperature of at least 600 ° C - this is an indicator for the lowest-calorie species. The ignition requires the use of wood to create the necessary heat.
Anthracite is primarily an industrial fuel. Its use in a furnace or boiler is irrational and expensive. In addition to high heat transfer, the advantages of anthracite include low ash content and low smoke content.
Charcoal is classified as a separate category as it is not a fossil fuel, but a product of production.
To obtain it, wood is treated in a special way in order to change its structure and remove excess moisture. The technology of obtaining an efficient and easy-to-use energy carrier has been known for a long time - before, wood was burned in deep pits, blocking the access of oxygen, but today special charcoal kilns are used.
Under normal storage conditions, the moisture content of charcoal is about 15%. Fuel ignites already when heated to 200 ° C. The specific calorific value of the energy carrier is high - it reaches 7400 kcal / kg.
The combustion temperature of charcoal varies depending on the type of wood and combustion conditions.


Burned wood fuel is economical - its consumption is much lower compared to using firewood. Besides high heat transfer, it is characterized by low ash content.
Due to the fact that charcoal burns with a small amount of ash and gives off an even heat without an open flame, it is ideal for cooking meat and other foods over an open fire. It can also be used for fireplace heating or cooking on a cooking stove.
Considering at what temperature a particular type of fuel burns, it should be borne in mind that figures are given that are achievable only under ideal conditions. In a home stove or solid fuel boiler, such conditions cannot be created, and it is not necessary. A brick or metal heat generator is not designed for this level of heating, and the coolant in the circuit will quickly boil.
Therefore, the combustion temperature of the fuel is determined by the mode of its combustion, that is, from the amount of air supplied to the combustion chamber.
Burning coal in a boiler
When burning an energy carrier in a boiler, it is impossible to allow the heat carrier to boil in the water jacket - if the safety valve does not work, an explosion will occur. In addition, a mixture of steam and water has a detrimental effect on the circulation pump in the heating system.
To control the combustion process, the following methods are used:
- the energy carrier is loaded into the furnace and the air supply is regulated;
- coal chips or fuel are dosed in pieces (according to the same scheme as in pellet boilers).
Combustion features
Coals differ in the type of flame. Burning coal and brown coal have long tongues of flame, anthracite and charcoal are short-flame energy sources. The short-flame fuel burns almost without residue, releasing a large amount of thermal energy.
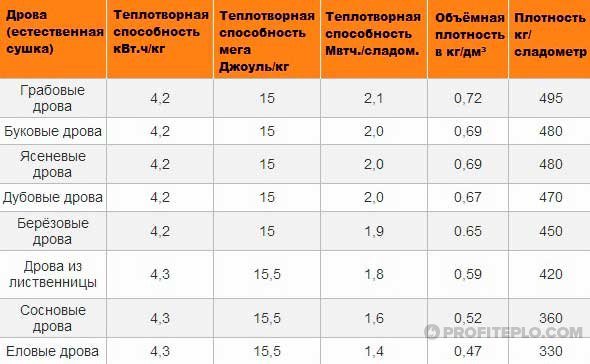

The combustion of long-flame energy carriers occurs in two stages. First of all, volatile fractions are released - a combustible gas that burns, rising to the top of the combustion chamber. In the process of gas evolution, coal is coked, and after the volatiles are burned out, the resulting coke begins to burn, forming a short flame. Carbon burns out, slags and ash remain.
When choosing which energy carrier is better to use for a solid fuel boiler or stove, you should pay attention to fossil fuels and charcoal. The combustion temperature is not critical, since in any case it will have to be limited in order to maintain the optimal operating mode of the heat generator.
Combustion - gasoline
Combustion of gasoline with detonation is accompanied by the appearance of sharp metal knocks, black smoke on the exhaust, an increase in gasoline consumption, a decrease in engine power and other negative phenomena.
The combustion of gasoline in the engine also depends on the excess air ratio. At the values a 0 9 - j - 1 1, the rate of pre-flame oxidation processes in the working mixture is the highest. Therefore, at these values of a, the most favorable conditions are created for the onset of detonation.
After the combustion of gasoline, the total mass of such pollutants increased significantly along with the general redistribution of their quantities. The percentage of benzene in the condensate of automobile exhaust gases was about 1 to 7 times higher than that in gasoline; the toluene content was 3 times higher, and the xylene content 30 times higher. It is known that oxygen compounds are formed in this case, and the number of ions characteristic of heavier unsaturated compounds of the olefin or cycloparaffin series and acetylene or diene series, especially the latter, increases sharply. Generally speaking, the changes to the Haagen-Smit chamber resembled the changes needed to make the composition of typical vehicle exhaust samples similar to those of the Los Angeles smog sample.
The calorific value of gasoline depends on its chemical composition.Therefore, hydrocarbons rich in hydrogen (for example, paraffinic ones) have a large mass heat of combustion.
Gasoline combustion products expand in the internal combustion engine along the polytrope n1 27 from 30 to 3 at. The initial temperature of gases is 2100 C; the mass composition of combustion products of 1 kg of gasoline is as follows: CO23 135 kg, H2 1 305 kg, O20 34 kg, N2 12 61 kg. Determine the work of expansion of these gases, if 2 g of gasoline is fed into the cylinder at the same time.
| Influence of TPP on carbon formation in the engine. |
When gasoline is burned from a thermal power plant, carbon deposits are formed that contain lead oxide.
When gasoline is burned in reciprocating internal combustion engines, almost all of the products formed are carried away with the exhaust gases. Only a relatively small part of the products of incomplete combustion of fuel and oil, a small amount of inorganic compounds formed from elements introduced with fuel, air and oil, are deposited in the form of carbon deposits.
When gasoline burns with tetraethyl lead, lead oxide is apparently formed, which melts only at a temperature of 900 C and can evaporate at a very high temperature, exceeding the average temperature in the engine cylinder. To prevent the deposition of lead oxide in the engine, special substances are introduced into the ethyl fluid - scavengers. The halogenated hydrocarbons are used as scavengers. Usually these are compounds containing bromine and chlorine, which also burn and bind lead in new bromide and chloride compounds.
| Influence of TPP on carbon formation in the engine. |
When gasoline is burned from a thermal power plant, carbon deposits are formed that contain lead oxide.
During the combustion of gasoline containing pure TPP, a plaque of lead compounds is deposited in the engine. The composition of the ethyl liquid grade R-9 (by weight): tetraethyl lead 54 0%, bromoethane 33 0%, monochloronaphthalene 6 8 0 5%, filler - aviation - gasoline - up to 100%; dye dark red 1 g per 1 kg of the mixture.
When gasoline containing TPP is burned, fistula oxide with low volatility is formed in the engine; Since the melting point of lead oxide is quite high (888), part of it (about 10%, counting on lead introduced with gasoline) is deposited as a solid residue on the walls of the combustion chamber, candles and valves, which leads to a rapid engine failure.
When gasoline is burned in a car engine, smaller molecules are also formed and the released energy is distributed in a larger volume.
Gases incandescent from the combustion of gasoline flow around the heat exchanger 8 (inside from the side of the combustion chamber and further, through the windows 5 outside, passing through the exhaust gas chamber 6) and heat the air in the heat exchanger channel. Next, hot exhaust gases are fed through the exhaust pipe 7 under the sump and heat up the engine from the outside, and hot air from the heat exchanger is fed through the breather into the crankcase and heats up the engine from the inside. In 1 5 - 2 minutes after the start of heating, the glow plug is switched off and combustion in the heater continues without its participation. After 7 - 13 minutes from the moment of receiving a pulse to start the engine, the oil in the crankcase warms up to a temperature of 30 C (at an ambient temperature of up to -25 C) and the unit starts pulses, after which the heater is turned off.
Combustion - oil product
Combustion of oil products in the embankment of the tank farm is eliminated by the immediate supply of foam.
Combustion of oil products in the embankment of the tank farm is eliminated by immediate supply of foam.
During the combustion of petroleum products, their boiling point (see Table 69) gradually increases due to the ongoing fractional distillation, in connection with which the temperature of the upper layer also rises.
| K Diagram of a fire-fighting water supply system for cooling a burning tank through an irrigation ring .. |
When burning oil in the tank, the upper part of the upper belt of the tank is exposed to the flame.When burning oil at a lower level, the height of the free side of the tank in contact with the flame can be significant. In this mode of combustion, the reservoir may collapse. Water from fire nozzles or from stationary irrigation rings, getting on the outer part of the upper walls of the tank, cools them (Fig. 15.1), thus preventing an accident and spreading of oil into the embankment, creating more favorable conditions for the use of air-mechanical foam.
The results of studying the combustion of petroleum products and their mixtures are interesting.
Its temperature during the combustion of oil products is: gasoline 1200 C, tractor kerosene 1100 C, diesel fuel 1100 C, crude oil 1100 C, fuel oil 1000 C. When burning wood in stacks, the temperature of the turbulent flame reaches 1200 - 1300 C.
Particularly large studies in the field of physics of combustion of petroleum products and their extinguishing have been carried out over the past 15 years at the Central Research Institute of Fire Defense (TsNIIPO), the Energy Institute of the USSR Academy of Sciences (ENIN) and a number of other research and educational institutes.
An example of negative catalysis is the suppression of the combustion of petroleum products with the addition of halogenated hydrocarbons.
Water promotes foaming and the formation of emulsions during the combustion of petroleum products with a flash point of 120 C and higher. The emulsion, covering the surface of the liquid, isolates it from the oxygen in the air, and also prevents the escape of vapors from it.
| Combustion rate of liquefied hydrocarbon gases in isothermal tanks. |
Combustion of liquefied hydrocarbon gases in isothermal tanks does not differ from the combustion of petroleum products. The combustion rate in this case can be calculated by formula (13) or determined experimentally. The peculiarity of the combustion of liquefied gases under isothermal conditions is that the temperature of the entire mass of liquid in the tank is equal to the boiling point at atmospheric pressure. For hydrogen, methane, ethane, propane and butane, these temperatures are, respectively, - 252, - 161, - 88, - 42 and 0 5 C.
| Installation diagram of the GVPS-2000 generator on the tank. |
Research and practice of extinguishing fires have shown that in order to stop the combustion of an oil product, the foam must completely cover its entire surface with a layer of a certain thickness. All foams with a low expansion rate are ineffective in extinguishing fires of oil products in tanks at the lower level of flooding. Foam, falling from a great height (6 - 8 m) onto the surface of the fuel, is dipped and enveloped in a film of fuel, burns out or quickly collapses. Only foam with a multiplicity of 70 - 150 can be thrown into a burning tank with hinged jets.
| Fire breaks. |
How draft in the stove affects combustion
If an insufficient amount of oxygen enters the furnace, then the intensity and temperature of wood combustion decreases, and at the same time its heat transfer decreases. Some people prefer to cover the blower in the stove in order to extend the burning time of one bookmark, but as a result, the fuel burns with a lower efficiency.

If firewood is burned in an open fireplace, then oxygen freely enters the firebox. In this case, the draft depends mainly on the characteristics of the chimney.
C 2H2 2O2 = CO2 2H2O Q (heat energy).
This means that when oxygen is available, the combustion of hydrogen and carbon occurs, which results in heat energy, water vapor and carbon dioxide.
For the maximum combustion temperature of dry fuel, about 130% of the oxygen required for combustion must enter the furnace. When the inlet flaps are closed, excess carbon monoxide is generated due to lack of oxygen. Such unburned carbon escapes into the chimney, but inside the furnace the combustion temperature drops and the heat transfer of the fuel decreases.
Modern solid fuel boilers are very often equipped with special heat accumulators. These devices accumulate an excessive amount of thermal energy generated during the combustion of fuel, provided there is good traction and high efficiency. This way you can save fuel.
In the case of wood-burning stoves, there are not so many opportunities to save firewood, since they immediately release heat into the air. The stove itself is capable of retaining only a small amount of heat, but the iron stove is not capable of this at all - excess heat from it immediately goes into the chimney.
So, with an increase in the thrust in the furnace, it is possible to achieve an increase in the intensity of fuel combustion and its heat transfer. However, in this case, heat loss increases significantly. If you ensure the slow combustion of wood in the stove, then their heat transfer will be less, and the amount of carbon monoxide will be more.
Please note that the efficiency of a heat generator directly affects the efficiency of burning wood. So, a solid fuel boiler boasts 80% efficiency, and a stove - only 40%, and its design and material matter.


The burning temperature of wood in the stove depends not only on the type of wood. The moisture content of the wood and the traction force, which is due to the design of the heating unit, are also significant factors.